User:Uzoma Anele
From Proteopedia
| Line 33: | Line 33: | ||
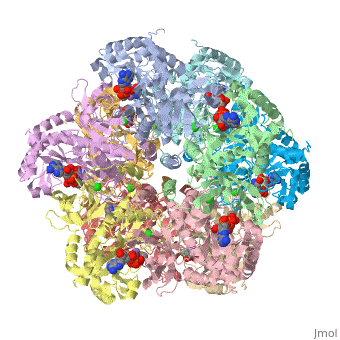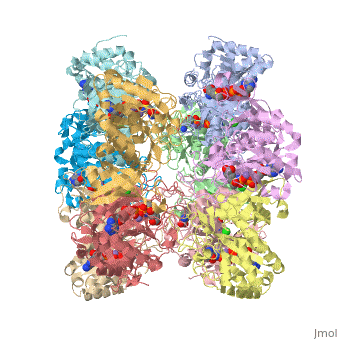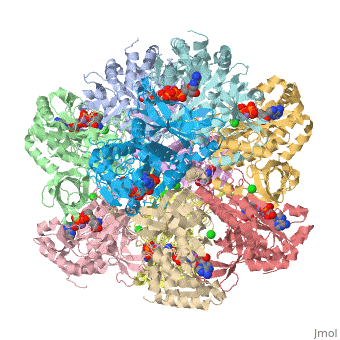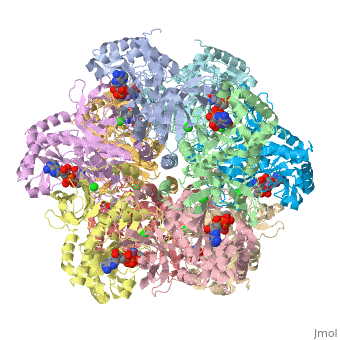
| - | Helical thongs provide a significant amount of intersubunit stabilization to the quaternary structure of glutamine synthetase by binding together subunits from the two layers. There exist 12 helical thongs in glutamine synthetase, each extending from each subunit. A typical <scene name='User:Uzoma_Anele/Helical_thong_2/1' target='1'>helical thong</scene>, shown here in red, is composed of a nonpolar carboxyl terminus (in this case of subunit A) that inserts into a <scene name='User:Uzoma_Anele/Helical_thong_pocket/1'>hydrophobic pocket</scene> created by two adjoining subunits (in this case subunits G and L) on the opposite, corresponding layer. An example of the <scene name='User:Uzoma_Anele/Helical_thong_complex/1'>helical thong complex</scene> details the C-terminal helix in red and the hydrophobic pocket in blue. Thirty-seven of the 77 residues of which the helical thong and hydrophobic pocket consist, are <scene name='User:Uzoma_Anele/Helical_thong_complex_polarity/2'>apolar</scene> (shown in gray) and are involved in the direct contact between the helical thong and pocket. Approximations of the stabilizing free eneregy created by these hydrophobic interactions suggest that helical thongs rely much more on hydrophobic interactions than hydrogen bonds. These residues contain an atypical abundance of <scene name='User:Uzoma_Anele/Helical_thong_complex_residues/1'>proline and valine</scene>, 16% and 10%, respectively (with proline shown in pink and valine in green). This unusual amount may serve to enhance its rigidity and cohesion. The helical thong complex can be seen in relation to the <scene name='User:Uzoma_Anele/Helical_thong_complex_gs/1'>entire enzyme</scene> with the helical thong of the A subunit shown in blue, and the hydropobic pockets created by the G and L subunits shown in yellow and | + | Helical thongs provide a significant amount of intersubunit stabilization to the quaternary structure of glutamine synthetase by binding together subunits from the two layers. There exist 12 helical thongs in glutamine synthetase, each extending from each subunit. A typical <scene name='User:Uzoma_Anele/Helical_thong_2/1' target='1'>helical thong</scene>, shown here in red, is composed of a nonpolar carboxyl terminus (in this case of subunit A) that inserts into a <scene name='User:Uzoma_Anele/Helical_thong_pocket/1'>hydrophobic pocket</scene> created by two adjoining subunits (in this case subunits G and L, shown in yellow and teal, respectively) on the opposite, corresponding layer. An example of the <scene name='User:Uzoma_Anele/Helical_thong_complex/1'>helical thong complex</scene> details the C-terminal helix in red and the hydrophobic pocket in blue. Thirty-seven of the 77 residues of which the helical thong and hydrophobic pocket consist, are <scene name='User:Uzoma_Anele/Helical_thong_complex_polarity/2'>apolar</scene> (shown in gray) and are involved in the direct contact between the helical thong and pocket. Approximations of the stabilizing free eneregy created by these hydrophobic interactions suggest that helical thongs rely much more on hydrophobic interactions than hydrogen bonds. These residues contain an atypical abundance of <scene name='User:Uzoma_Anele/Helical_thong_complex_residues/1'>proline and valine</scene>, 16% and 10%, respectively (with proline shown in pink and valine in green). This unusual amount may serve to enhance its rigidity and cohesion. The helical thong complex can be seen in relation to the <scene name='User:Uzoma_Anele/Helical_thong_complex_gs/1'>entire enzyme</scene> with the helical thong of the A subunit shown in blue, and the hydropobic pockets created by the G and L subunits shown in yellow and teal, respectively.<ref name="review">Eisenberg, D., et.al., Structure-function relationships of glutamine synthetases, Biochim Biophys Acta 2000: 1477, 122-145.</ref><ref name="struct">Yamashita, M. M., ''et.al.,'' Refined Atolnic Model of Glutamine Synthetase at 3.5 A Resolution, J Biol Chem 1989 264: 17681-17690.</ref> |
<applet load='2gls' size='500' frame='true' align='center' caption='Salmonella typhimurium Glutamine Synthetase' /> | <applet load='2gls' size='500' frame='true' align='center' caption='Salmonella typhimurium Glutamine Synthetase' /> | ||
Revision as of 23:05, 7 December 2008
Uzoma Anele, Sandeep Pulugurtha Undergraduate students at the University of Maryland, Baltimore County.
Contents |
Exercise 1
|
Exercise 2
Exercise 3
Exercise 4
Outline
Helical thongs provide a significant amount of intersubunit stabilization to the quaternary structure of glutamine synthetase by binding together subunits from the two layers. There exist 12 helical thongs in glutamine synthetase, each extending from each subunit. A typical , shown here in red, is composed of a nonpolar carboxyl terminus (in this case of subunit A) that inserts into a created by two adjoining subunits (in this case subunits G and L, shown in yellow and teal, respectively) on the opposite, corresponding layer. An example of the details the C-terminal helix in red and the hydrophobic pocket in blue. Thirty-seven of the 77 residues of which the helical thong and hydrophobic pocket consist, are (shown in gray) and are involved in the direct contact between the helical thong and pocket. Approximations of the stabilizing free eneregy created by these hydrophobic interactions suggest that helical thongs rely much more on hydrophobic interactions than hydrogen bonds. These residues contain an atypical abundance of , 16% and 10%, respectively (with proline shown in pink and valine in green). This unusual amount may serve to enhance its rigidity and cohesion. The helical thong complex can be seen in relation to the with the helical thong of the A subunit shown in blue, and the hydropobic pockets created by the G and L subunits shown in yellow and teal, respectively.[1][2]
|