We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Doppel
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
Dpl has the same fold as PrP, with three alpha helices and two short beta strands<ref>Mo H ''et al.'' (2001) ''Proc. Natl. Acad. Sci. USA'' '''98''',2352-7</ref>. however it differs in that the third helix has a significant kink in it and it also contains two disulphide bonds. | Dpl has the same fold as PrP, with three alpha helices and two short beta strands<ref>Mo H ''et al.'' (2001) ''Proc. Natl. Acad. Sci. USA'' '''98''',2352-7</ref>. however it differs in that the third helix has a significant kink in it and it also contains two disulphide bonds. | ||
| - | The structure mutant PrP with the additional disulphide bond was also determoned <ref>Zahn R ''et al.'' (2003) NMR structure of a variant human prion protein with two disulfide bridges '' J. Mol. Biol.'' '''326''', 225-34. | + | The structure mutant PrP with the additional disulphide bond was also determoned <ref>Zahn R ''et al.'' (2003) NMR structure of a variant human prion protein with two disulfide bridges '' J. Mol. Biol.'' '''326''', 225-34.</ref> |
| - | =Related structures= | + | ==Related structures== |
* [[1z65]] Mouse Dpl residues 1-30 | * [[1z65]] Mouse Dpl residues 1-30 | ||
* [[1lg4]] Human Dpl residues 24-152 | * [[1lg4]] Human Dpl residues 24-152 | ||
| Line 16: | Line 16: | ||
| - | =References= | + | ==References== |
</reference> | </reference> | ||
Revision as of 09:20, 15 December 2008
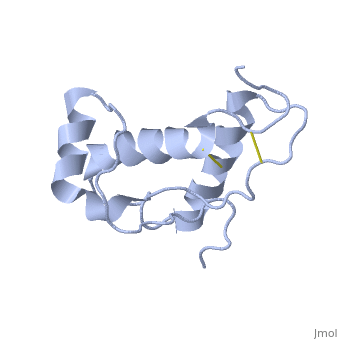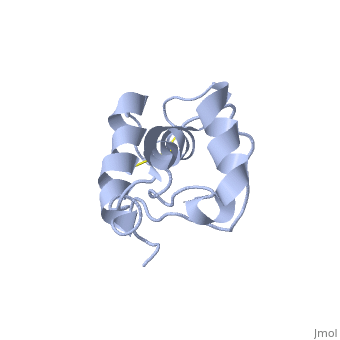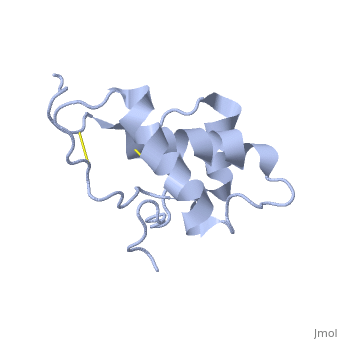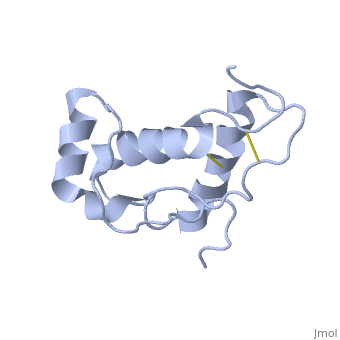
Doppel (Dpl), named for downstream prion protein-like[1], is a homolog of the prion protein (PrP). It is a cell surface glycoprotein.
Structure of Dpl
| |||||||||
| 1lg4, 20 NMR models () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene: | Prnd (Homo sapiens) | ||||||||
| Related: | 1i17 | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Dpl has the same fold as PrP, with three alpha helices and two short beta strands[2]. however it differs in that the third helix has a significant kink in it and it also contains two disulphide bonds.
The structure mutant PrP with the additional disulphide bond was also determoned [3]
Related structures
- 1z65 Mouse Dpl residues 1-30
- 1lg4 Human Dpl residues 24-152
- 1i17 Mouse Dpl residues 51-157
- 1h0l Human PrP residues 121-230, with an additional disulphide bond analogous to the homolog Doppel
References
</reference>