Resolution
From Proteopedia
(polishing) |
(polishing) |
||
| Line 21: | Line 21: | ||
[http://proteopedia.org/wiki/images/1/1a/Resolution_holton.mpeg '''PLAY MOVIE'''] | [http://proteopedia.org/wiki/images/1/1a/Resolution_holton.mpeg '''PLAY MOVIE'''] | ||
</big></center> | </big></center> | ||
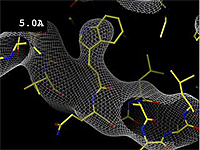
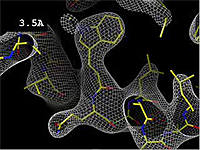
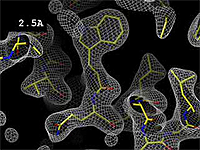
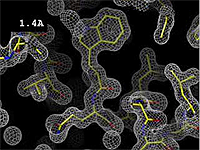
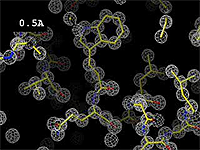
| - | At 0.5 Å, every atom<ref name="stills" /> of the tryptophan sidechain in the top center of the | + | At 0.5 Å in the movie, every atom<ref name="stills" /> of the tryptophan sidechain in the top center of the frame is clearly represented by a sphere of electron density. At 2.5 Å (a bit worse than the median in the [[PDB]]), the overall shape and position of the Trp sidechain is still clear, as is the alpha helical conformation of the main chain. However, at 5.0 Å, only an ill-fitting bump is present to signal the bulky Trp sidechain, and the alpha helix becomes a cylinder of electron density, from which the handedness of the helix may not be discernable. |
==See Also== | ==See Also== | ||
Revision as of 19:04, 3 January 2009
Resolution is an average value for the uncertainty of atomic positions in a crystallographic model. High values for resolution (e.g. 5.0 Å) mean high uncertainty, and low values (e.g. 1.0 Å) mean much less uncertainty. 2.05 Å is the median resolution for X-ray crystallographic results in the Protein Data Bank (43,066 on May 2, 2008).
The uncertainty for each atom is quantitated in its temperature value.
The images at right show how the electron density map[1] becomes more accurate and detailed as the uncertainty (resolution value) decreases from 5.0 Å to 0.5 Å.
The images at right were taken from a movie[2] in which the atomic model and electron density map rock back and forth while the resolution value (uncertainty) increases from 0.5 to 5.0 Å.
At 0.5 Å in the movie, every atom[1] of the tryptophan sidechain in the top center of the frame is clearly represented by a sphere of electron density. At 2.5 Å (a bit worse than the median in the PDB), the overall shape and position of the Trp sidechain is still clear, as is the alpha helical conformation of the main chain. However, at 5.0 Å, only an ill-fitting bump is present to signal the bulky Trp sidechain, and the alpha helix becomes a cylinder of electron density, from which the handedness of the helix may not be discernable.
See Also
Websites
- Resolution at ProteinExplorer.Org's Glossary.
Notes
- ↑ 1.0 1.1 These are "perfect" electron density maps calculated from the atomic model (R factor = 0.0%, perfect phases and amplitudes, contoured at 1 sigma). Electron density maps based on experimental data would fit the true conformation less well. Because these electron density maps were calculated from an atomic model that lacked hydrogen atoms, the electron densities for hydrogen atoms that would appear with experimental data at a resolution of 0.5 Å do not appear.
- ↑ The movie (Image:Resolution holton.mpeg) was created by James Holton at the Advanced Light Source of the Berkeley Laboratory at the University of California. Holton gave explicit permission to use this movie in Proteopedia. The original source was http://ucxray.berkeley.edu/~jamesh/movies.
Proteopedia Page Contributors and Editors (what is this?)
Eric Martz, Joel L. Sussman, Wayne Decatur, Eran Hodis, YongLiang Jiang, Jaime Prilusky