General Structure
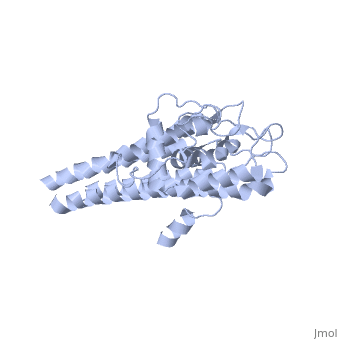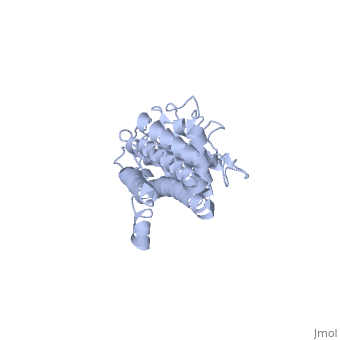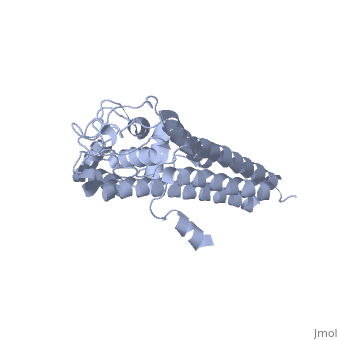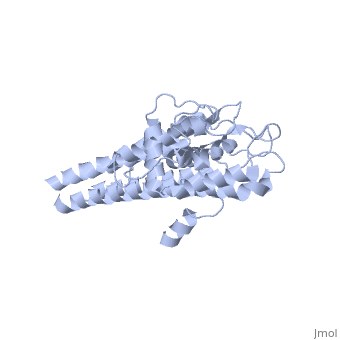
There are two distinct classes of HMGRs, class I HMGRs, which are only found in eukaryotes and are membrane bound and class II HMGRs, which are found in prokaryotes and are soluble.[1] HMGR contains 8 transmembrane domains that have yet to be successfully crystallized, which anchor the protein to the membrane of the endoplasmic reticulum.[2] The catalytic portion of human HMGR forms a tetramer, with the individual monomers winding around each other.[2] Within the tetramer, the monomers are arranged into , each of which contains which are formed by residues form both monomers. Each monomer contains , the , the , and the . The L-domain is unique to HMGRs while the S-domain, which forms the binding site for NADP, resembles that of ferredoxin. The S and L domains are connected by a which is essential for the HMG-binding site.[2] Salt bridges between residues R641 and E782 as well as between E700 and E700 on neighboring monomers compliment the largely hydrophobic dimer-dimer interface.[2]