MRGPRX2 is a certain type of GPCR that is located in the cellular membranes of mast cells.
Background
GCPR’s or G-Protein Coupled Receptors are a special type of protein receptor that promotes cellular signaling. Due to its structure spanning the cellular membrane, it works to transmit extracellular signals to create a change inside the cell. This is called signal transduction, and is a common way extracellular signals produce a change within cells. Some common cellular pathways that utilize GPCR’s are found in Rhodopsin, a protein essential for the human vision response, or the adrenaline fight-or-flight response. Understanding GPCR’s and how they produce their desired intracellular signal is essential understanding essential cellular pathways, especially in their diseased states. As of 2017, there were about 475 drugs in circulation that acted on 108 GPCR targets, and at least 321 GPCR-targeting drugs were in clinical trial stages, with about 20% of them targeting novel GPCR’s. [1] Because of the clinical relevance of GPCR’s, every new structure found, such as MRGPRX2, contributes to essential drug development to both treat disease, or modulate harmful side effects.
Because of how many different types of GPCR’s there are, they have been categorized into 6 different classes based on shared sequences and functions.[2] MRGPRX2 is categorized into the Class A receptor family, however it has important differences that make it a unique type of Class A receptor. [3] [4]
GPCR Structure
Cryo-electron microscopy (cryo-EM) was used to image the MRGPRX2 receptor to analyze its structure. [3] [4] which helped to classify it into the A family of GPCR's. MRGPRX2 therefore shares the same general structural domains of all GPCR's. This includes a and a domain. The G-protein domain consists of , , and subunits.
Transmembrane Domain
The transmembrane domain spans the cellular membrane. It consists of seven transmembrane helices, three extracellular loops, and three intracellular loops. The transmembrane helices are numbered 1-7 and contain special conserved motifs that are shared across other A family receptors. These motifs are expanded upon later, as they heavily contribute to the structure and therefore function of the transmembrane domain as a whole.
The extracellular domain of the protein is responsible for ligand binding, which initiates signal transduction. The properties of the extracellular environment of the transmembrane domain determines what ligands bind to the protein, and what type of binding interactions the protein and ligand have. In MRGPRX2, the extracellular side of the protein has two binding pockets. Binding pocket one is positively charged due to positively charged lysine residues, while binding pocket two contains hydrophobic amino acids, which contribute to hydrophobic interactions between ligand and protein.
The intracellular domain is what connects the transmembrane and G-protein domains together. There are a wide variety of residues and important interactions that contribute to this interaction, and it is very important in transmitting the extracellular signal of ligand binding to the intracellular environment where the G-protein binds and can become activated.
G-Protein
Novel Characteristics
The most important findings about the MRGPRX2 receptor are the differences between it and all other previously discovered GPCR's found in the A family. Many conserved structural motifs, characteristic of the A family receptors, are absent on MRGPRX2, which contribute significantly to the different ligand interactions it has.
Toggle Switch
Previously known Tryptophan residue that acts as the Toggle Switch in GPCR is switched to a .
Sodium Site
PIF/LLF Motif
LLF Motif on .
DRY/ ERC Motif
Disulfide Bonds
Disulfide bond connecting .
Ligands
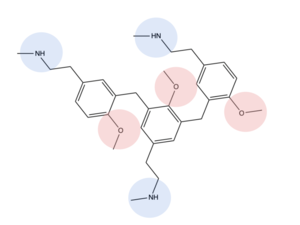
C48/80 ligand description
Function
Before Activation
After Activation
Clinical Relevance
3D Structures
</Structure Section>
References
- ↑ Cite error: Invalid
<ref> tag;
no text was provided for refs named Hauser
- ↑ Cite error: Invalid
<ref> tag;
no text was provided for refs named Basith
- ↑ Cite error: Invalid
<ref> tag;
no text was provided for refs named Cao
- ↑ Cite error: Invalid
<ref> tag;
no text was provided for refs named Yang
[1]

