Leucine-rich repeat
From Proteopedia
The leucine-rich repeat proteins are a large family of over 60,000 proteins found in viruses, bacteria, archaea, and eukaryotes that feature horseshoe- or arc-shaped domains made of leucine-rich repeating motifs.[1]
| |||||||||||
| |||||||||||
<slideshow sequence="random" transition="fade" align="left" refresh="5500">
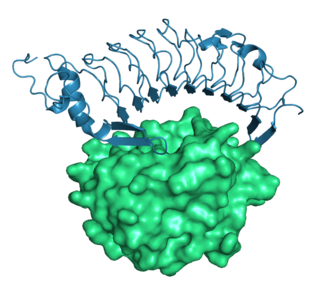
The human leucine-rich repeat family member Glycoprotein Ib alpha (blue) involved in Willebrand disease bound to the von Willebrand Factor A1 Domain (green surface), from 1m10.
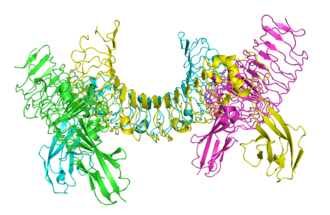
Lingo-1 protein involved in inhibiting effective regrowth of axons after central nervous system damage, from 2id5.
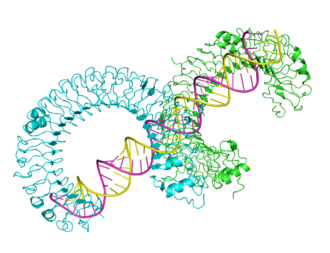
Mouse Toll-like receptor bound to dsRNA, from 3ciy.
</slideshow>
| |||||||||||
| |||||||||||
Articles in Proteopedia concerning Leucine-rich repeat proteins include:
- Human Follicle Stimulating Hormone Complexed with its Receptor
- Variable Lymphocyte Receptors
- Lamprey Variable Lymphocyte Receptor
- Toll-like receptors (TLRs), such as Mouse Toll-like receptor bound to dsRNA
- Complex of Glycoprotein Ib alpha and the von Willebrand Factor A1 Domain
- Lingo-1 protein involved in inhibiting effective regrowth of axons after central nervous system damage
To view automatically seeded indices concerning Leucine-rich repeat proteins[2], see:
- Leucine-rich repeat
- Leucine-rich-repeat
- Leucine-rich repeat-containing protein 4
- LRR
- Leucine-rich repeat glycoprotein
References
- ↑ Matsushima N, Miyashita H, Mikami T, Kuroki Y. A nested leucine rich repeat (LRR) domain: the precursor of LRRs is a ten or eleven residue motif. BMC Microbiol. 2010 Sep 9;10:235. PMID:20825685 doi:10.1186/1471-2180-10-235
- ↑ Matsushima N, Miyashita H, Mikami T, Kuroki Y. A nested leucine rich repeat (LRR) domain: the precursor of LRRs is a ten or eleven residue motif. BMC Microbiol. 2010 Sep 9;10:235. PMID:20825685 doi:10.1186/1471-2180-10-235
See Also
Additional Literature
- Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001 Dec;11(6):725-32. PMID:11751054
- Matsushima N, Tanaka T, Enkhbayar P, Mikami T, Taga M, Yamada K, Kuroki Y. Comparative sequence analysis of leucine-rich repeats (LRRs) within vertebrate toll-like receptors. BMC Genomics. 2007 May 21;8:124. PMID:17517123 doi:10.1186/1471-2164-8-124
- Matsushima N, Tachi N, Kuroki Y, Enkhbayar P, Osaki M, Kamiya M, Kretsinger RH. Structural analysis of leucine-rich-repeat variants in proteins associated with human diseases. Cell Mol Life Sci. 2005 Dec;62(23):2771-91. PMID:16231091 doi:10.1007/s00018-005-5187-z
- Kajava AV, Kobe B. Assessment of the ability to model proteins with leucine-rich repeats in light of the latest structural information. Protein Sci. 2002 May;11(5):1082-90. PMID:11967365 doi:10.1110/ps.4010102
- Jin MS, Lee JO. Application of hybrid LRR technique to protein crystallization. BMB Rep. 2008 May 31;41(5):353-7. PMID:18510864
- Carpenter S, O'Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem J. 2009 Jul 29;422(1):1-10. PMID:19627256 doi:10.1042/BJ20090616
