Type I
Type I interferons are homologous helical cytokines that effect a wide variety of cells pleiotropically. These effects range from antiviral activity to antibacterial, antiprozoal, immunodulatory, and cell growth regulatory functions. Without Type I interferons, the survival of the higher vertebrates would be impossible. Because of their strong antiviral and antiproliferative effects, these interferons are used in the treatment of numerous cancers, hepatitis C, and multiple sclerosis.
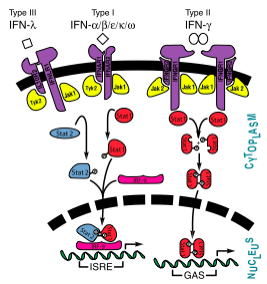
All type I interferons bind to a cell surface receptor consisting of two subunits: IFNAR1 and IFNAR2. These receptors belong to a class II helical cytokine receptor family (HCRII). Other members of this family include the interferon-γ receptor (IFNGR), tissue factor (TF), the interleukin 10 receptor (IL20R1 and IL20R2), IL-28BP, IFNLR, and IL28Rα.[3]
Interferon-α
Interferon alpha has a 31% sequence homology to interferon beta. It, too, has many with two : one between the , and the other between . It has seven , as compared to the five of interferon-β, and therefore has several more The helices A, C, and F run to one another, and to B, E, and G which run to each other.
does not run parallel or anti-parallel to either set, but rather runs at a 45-90 degree angle to them. Helix A consists of residues 10-12; Helix B of 40-43; Helix C of 53-68; Helix D of 70-75; Helix E of 78-100; Helix F of 109-132; and Helix G of 137-158.
Interferon-α to an interferon receptor mainly with helices C and G. There are many within 4 angstroms of one another. These residues could form many , illustrated in white dotted lines. Given that interferon-α does not undergo many structural changes upon binding to interferon receptor II, Quadt-Akabayov et al. have concluded that the binding mechanism is similar to that of a lock and key. While interferon-α and -β bind to the same receptors as one another, the affinities with which they bind to IFNAR1 and IFNAR2 differ. While the binding to IFNAR2 is stronger for both in comparison to IFNAR1, interferon-β has a much stronger affinity for IFNAR1 than interferon-α.[4]
Interferon-β
is a protein growth factor that stimulates an antiviral defense. Its encoding gene is one of only two known vertebrate structural genes that lacks introns.[5]
Interferon-β is a relatively simple biological response modifier, with several . It consists of five , as well as multiple interconnecting . Helices A, B and D run , and helices C and E run to the other three helices, but to one another. Helix A consists of residues 6-23; Helix B consists of residues 49-65; Helix C consists of residues 77-91; Helix D consists of residues 112-131; and Helix E consists of residues 135-155.[6][7]
Interferons -α and -β interact with a receptor at the cell surface.[8] This receptor has : an
, with two disulfide bonds, a , with one disulfide bond, and a . The of the receptor have no secondary structure, allowing for some serious flexibility, leading to .[9]
Interferon-ε
Interferon-κ
Interferon-ω
Type II
Interferon-γ