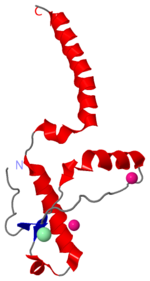
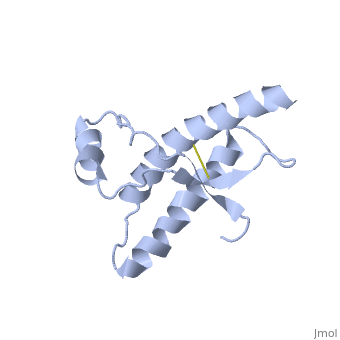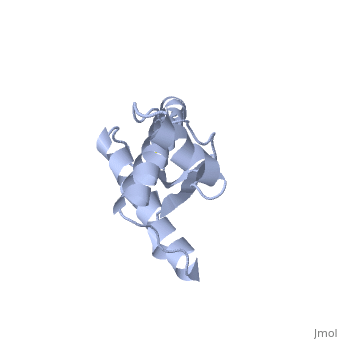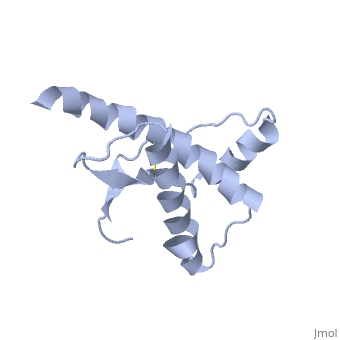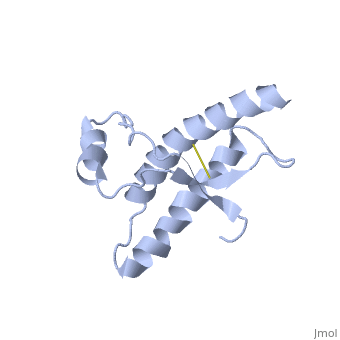
The specific residues shown alter the electrostatic properties of the surface of PrPC, however their postions on the wild-type monomer and fully unfolded PrPSc, do not illustrate a clear mechanism for propagation. The dimer form clearly shows the reason for the infectious qualities of the variable and disease conforming residues.
It is theorized from this dimeric structure that the dimerization is the first step in amyloid formation and the presence of these dimers could possibly speed up the aggregation of PrPSc.
The following interactions and residue switching portray possible catalytic sites:
Residues 129, 200, and 164 to 170 are shown to exist right at the dimer interfaces (as noted in the previous structures). The placement of these residues within the dimer indicates the dimerization as the point of control for these specific residues.
Helix 1 (Ser 143−Tyr 157) exists at the dimer interface. [6]
Helix 2 (Asn 171−Thr 188) is linked to the C-terminal helix 3 (Thr 199−Tyr 225).
The switch region 189-198 is shown to be unfolded.
There are also additional intrachain van der Waals and electrostatic interactions between the switch region and helix 1 in the 3D domain-swapped dimeric crystal structure that are not possible in the monomeric NMR structure.
Hydrogen bonding between the dimers exist: Thr188 O−Gly195 N, Thr190 O−Lys194 N and Thr192 O−Thr192 N
Altered in familial: 119, 129, 226 shown
Asp 202 and Arg 220, which are located at either end of helix 3, form interchain hydrogen bonds that confer specificity to the interaction
Due to the importance of initial dimerization in the formation of prion aggregates, the step of dimerization presents a known step for treatments to target.