Sandbox Reserved 1123
From Proteopedia
Contents |
Introduction
Since 2000, 38,1 million people were infected by the human immunodeficiency virus (HIV)[1]. This virus can cause the acquired immunodeficiency symptom (AIDS) which can often be lethal. The HIV belongs to the retrovirus family, more precisely to the lentivirus. This virus infects immune cells (T cells, macrophages, …) by integrating its genome in the host cell genome. Since HIV is a RNA virus, it converts its RNA into DNA thanks to the reverse transcriptase. Nevertheless, when its genome is integrated in the host genome, it can stay in latency during a long time.
The virus becomes active when the cell starts to fight another infection. The host cell transcript its own DNA and the viral DNA. This viral DNA codes for lytic proteins and viral proteins. The lytic proteins will kill the host cell, thus releasing the new viral particules. Indeed, it is the immune cells death which induces the decrease of the immune response and enhances thus the sensibility to other infections. Moreover, the viral proteins will produce new viruses which will be released and infect new cells. In this way, the knowledge of the different viral proteins, like the capsid proteins, can allows us to find new targets in order to avoid the viral dispersion/propagation ? [2]
Function
Structural highlights
|
|
Structural basis of HIV-1 mature capsid
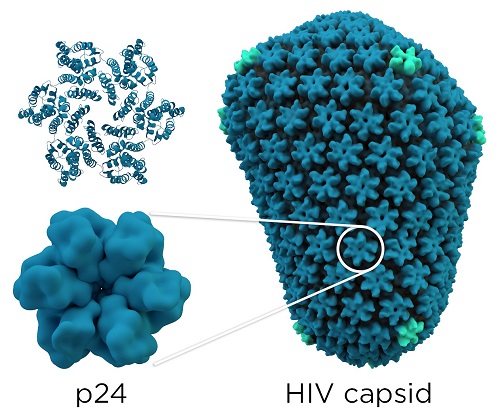
Each HIV-1 virus owns a conical core capsid that encapsulates the ssRNA(+) viral genome and some viral enzymes that are essential for the host infection. This capsid contains about 250 hexamers and 12 pentamers of the CA protein, pentamers are important to generate the curvature of the structure. This capsid is the mature capsid, its formation results in the maturation and disassembly of the immature gag polyproteins (first structural protein products which are encoded by the HIV-1 genome) by viral proteases. CA proteins are organized in two domains: an N-terminal domain ( ) and a C-terminal domain (). The mature capsid is formed by the assembly of approximately two-thirds of the mature CA proteins in the viral particle. CA proteins bind together and organize themselves in order to create a lattice of hexameric rings, these rings contain an inner ring of six s, surrounded by a belt of six s. Then, 12 CA pentamers join the structure, it permits the formation of a closed protein shell. [1]
Primary structure
CA proteins are composed of about 230 residues [4] and as we mentioned above they contain two domains: (in green) and (in yellow). These two domains are linked together thanks to a (in orange). [1]
Interactions with others partners
Even if p24 is classified as a structural protein, it is also involved in many cellular infection processes.
You can find bellow in a non exhaustive list of p24 partners :
- Cyclophylin A [5]
The HIV-1 capsid acts like a kind of "nuclear localisation signal" because it targets directly the virus toward the nucleus, where the integration takes place.
Capsid as therapeutical target
|
Thank to its central role in viral infectious process (genome protection, enveloppe cohesion, uncoating, budding), HIV capsid is a very good target for antiviral drugs. As a result, many research teams are working in this way and some molecules such as [6] are very promising. By binding to a "central pocket" on P24, it was shown in vitro that this compound is inhibiting the new viruses assembly, and consequently the virus budding.
This approach is interesting because, based on structural information, we are able to build such ligands using drug design. However, we are still far away from the "miraculous HIV drug", because the pathway from design to approved drug is not an easy way at all.
References
- ↑ Global HIV and AIDS statistics
- ↑ Weiss RA (May 1993). "How does HIV cause AIDS?". Science 260 (5112): 1273–9.Bibcode:1993 Science 260.1273W. doi:10.1126/science.8493571. PMID 8493571.
- ↑ Fernandez J, Gärtner K, Becker A, et al. HIV-1 capsid interacts with cytoskeletal-associated proteins for intracytoplasmic routing to the nucleus. Retrovirology. 2013;10(Suppl 1):P34. doi:10.1186/1742-4690-10-S1-P34.
- ↑ Fernandez J, Portilho DM, Danckaert A, Munier S, Becker A, Roux P, Zambo A, Shorte S, Jacob Y, Vidalain PO, Charneau P, Clavel F, Arhel NJ. Microtubule-associated proteins 1 (MAP1) promote human immunodeficiency virus type I (HIV-1) intracytoplasmic routing to the nucleus. J Biol Chem. 2015 Feb 20;290(8):4631-46. doi: 10.1074/jbc.M114.613133. Epub 2014 Dec 11.
- ↑ [http://jvi.asm.org/content/84/10/5181.long Marisa S. Briones, Charles W. Dobard and Samson A. Chow. Role of Human Immunodeficiency Virus Type 1 Integrase in Uncoating of the Viral Core. Accepted manuscript posted online 10 March 2010, doi:.1128/JVI.02382-09 J. Virol. May 2010 vol. 84 no. 10 5181-5190]
- ↑ 6.0 6.1 6.2 HIV-1 capsid: the multifaceted key player in HIV-1 infection Nature Reviews Microbiology 13,471–483 (2015)doi:10.1038/nrmicro3503
Structural capsid image : By Thomas Splettstoesser (www.scistyle.com) (Own work) [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons