This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function
Disease
Sickle Cell Anemia
For the most part, amino acid sequences can be slightly different, but sometimes a change in the sequence can have a large impact. For example, in sickle cell anemia, glutamate 6 in the beta chain is mutated to valine, resulting in a structural change that allows hemoglobin to stick to each other and create stiff fibers, which creates the sickle shape in blood cells.
Relevance
Structural highlights
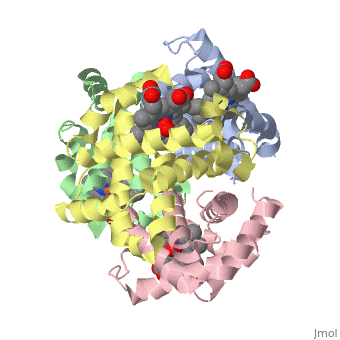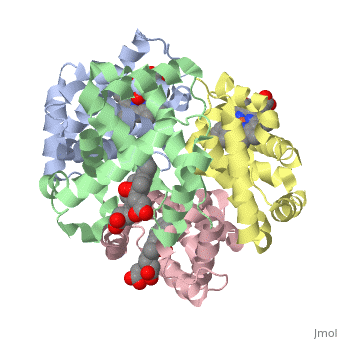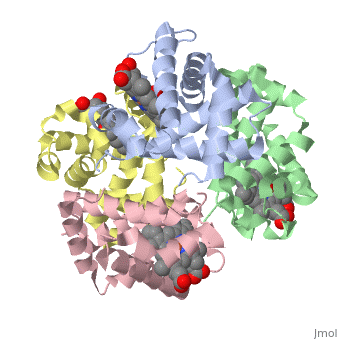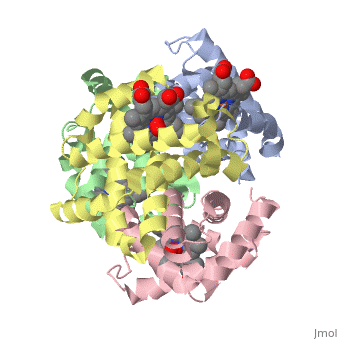
Hemoglobin is made of four protein chains. Each chain looks like myoglobin, which is used to store oxygen in muscles. Each individual chain has a heme group that has an iron atom that allows oxygen to bind. Of the chains, two are beta chains and two are alpha chains.
In the chain, the heme group has iron that allows the oxygen to bind.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.