Image:ANAAT-mechanism his catalysis.jpg
From Proteopedia
No higher resolution available.
ANAAT-mechanism_his_catalysis.jpg (701 × 502 pixel, file size: 38 KB, MIME type: image/jpeg)
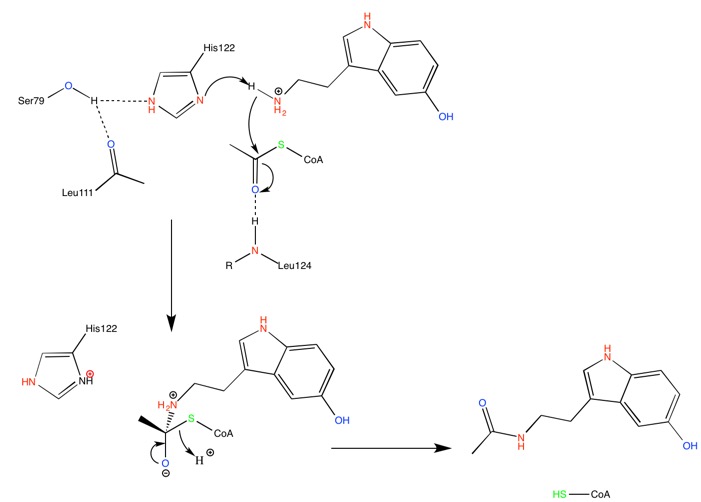
Putative Acid/base catalysis mechanism perfomed by imidazole groups of the two active site conserved His.
Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2.5A Resolution Suggests a Catalytic Mechanism. Mol. Cell. 1999, 3-1, 23-32.
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 14:05, 2 January 2019 | Petit Loup (Talk | contribs) | 701×502 | 38 KB | Putative acid/base catalysis mechanism performed by imidazole groups of the two active site conserved Histidines of ANAAT. Original picture. Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2 |
| 14:02, 2 January 2019 | Petit Loup (Talk | contribs) | 701×502 | 38 KB | Putative Acid/base catalysis mechanism perfomed by imidazole groups of the two active site conserved His. Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2.5A Resolution Suggests a Catalytic |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
