Introduction
SHOC2-PP1C-MRAS complex is a ternary holophosphatase complex formed by the individual proteins: SHOC2, PP1C, and MRAS. SHOC2-PP1C-MRAS complex is involved in cell proliferation because it functions as a regulator of RTK-RAS signaling by activating RAF through dephosphorization. Complex formation is initiated by ligand binding of receptor tyrosine kinase receptor(RTK). The RTK activates membrane bound MRAS by signaling the exchange of GDP for GTP. GTP bound MRAS initiates complex formation, this complex and is able to dephosphorylate the RAF complex leading to further downstream signaling effects.
Overall Structure
SHOC2
MRAS
Key Ligand Interactions
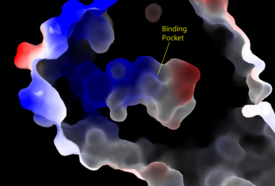
Figure 3: Electrostatic illustration of the amphipathic binding pocket of the LPA
1 receptor. This binding pocket was revealed by cutting away the exterior or the protein. This binding pocket, located in the interior of the protein, has both polar and nonpolar regions. The blue and red coloration highlight the positively and negatively charged regions, respectively, and the white color shows the nonpolar region of the binding pocket.
SHOC2 and PP1C
SHOC2 and MRAS
PP1C and MRAS
Signaling Pathway
Disease Relevance
Cancer
RASopathies
Future Studies
3D structures of lysophosphatidic acid receptor
4z34, 4z35, 4z36 - hLPA1 + antagonist - human
2lq4 – hLPA1 second extracellular loop – NMR
4p0c – hLPA2/NHERF2
5xsz – LPA6A (mutant) – zebra fish
References
Proteopedia Resources
Category:Lysophosphatidic acid binding
Category:Lysophosphatidic acid
Butler University Proteopedia Pages
See also:

