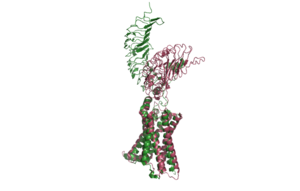This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
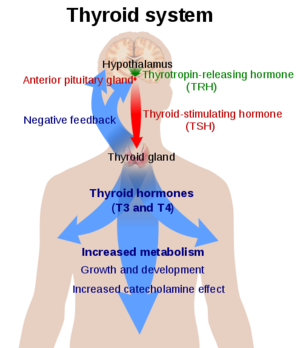
Thyroid Stimulating Hormone Receptor (TSHR) is a type of G-Protein Coupled Receptor (GPCR) found in human thyroid follicles. It is activated by the Thyroid Stimulating Hormone (TSH) which is known as thyrotropin. Activation of TSHR is neccessary for activating a signaling pathway for the production of thyroid hormones such as T3 and T4 (Fig 1).
Structure
TSHR forms a complex with TSH and Gs proteins. This is called the .
: Leucine Rich Region Domain (coral), the hinge region (blue-purple), and the transmembrane region(rainbow). The leucine rich region domain is extracellular. This is where TSH will bind. The hinge region is also extracellular. Conformational changes in this region are responsible for the switch between the active vs inactive state. Finally, the transmembrane region is located within the plasma membrane. Its function is to hold the receptor into the membrane. This domain is also bound to the [G-proteins https://en.wikipedia.org/wiki/G_protein] at the N-terminus. The G-proteins are located on the intracellular side of the plasma membrane. They are important for transmitting the binding signal into the cell, setting off a protein signaling cascade.
Transmembrane Region
() is embedded within the cell membrane. Like other G-protein receptors, it is made up of a 7-pass helix [3]. It is made up of about 284 residues. The transmembrane region is surrounded by a "belt" of . When cholesterol binding sites are mutated such that they are unfunctional, TSHR activity decreases. Thus, the cholesterols are important for TSHR function [4]. Additionally, at the N-terminus, the transmembrane region binds to the , which are located intracellularly.
Leucine Rich Domain
The is part of the extracellular region of TSHR. It is made up of about 280 different residues. Connected to its C-terminus is the Hinge Region. It is made up of an extensive parallel β-sheet. This β-sheet is where TSH binds and is called the binding pocket[4].
Hinge Region
The (purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. It is also sometimes referred to as the signaling specificity domain because there is some evidence suggesting that this region is important in both TSH binding and signal transduction. [5]. It is made up of two α-helices that are connected via di-sulfide bonds(shown in yellow) and is made up of about 134 residues. Interactions between these two helices and TSH help orient TSH properly. These interactions are essential for TSH binding, however, they are not required for the activation of TSHR. Conformational changes in this region, specifically the orientation of , are responsible for the bringing TSHR into the active state [3]
Active vs Inactive State
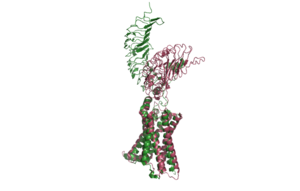
Figure 2: An overview of the Inactive (pink) vs Active (green) state of TSHR. PDB: 7WX5
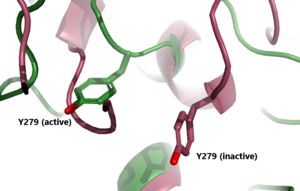
Figure 3: A zoomed in view of the Y279 residue in the Hinge Region of TSHR, showing the 6 angstrom move of Y279 during the activation of TSHR. Active TSHR is shown in green (PDB: 7t9i) and inactive TSHR is shown in pink (PDB: 7t9m).
When TSHR is not bound to TSH, it is in the . This is also considered the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashing between TSH and the cell-membrane cause TSHR to take on the (fig 2). During this transition, the Extracellular domains rotate 55° along an axis. This rotation is caused by conformational changes within the , specifically at the . This residue moves 6 angstroms relative to I486, which is a residue located in the Transmembrane Region [3] (Fig 3).
Specific Residues
Biological Relevance
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.