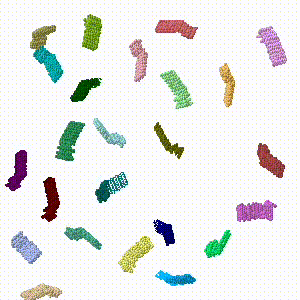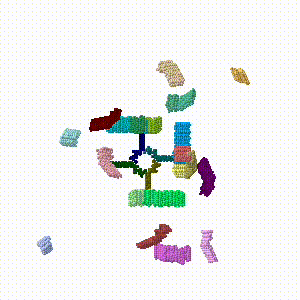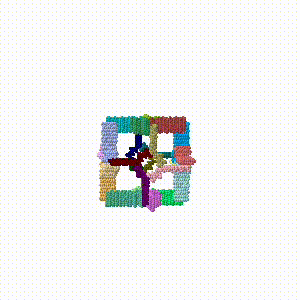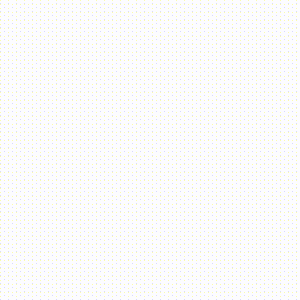Synthetic nanomaterials from standardized protein blocks
From Proteopedia
| |||||||||||
Method of simulating assembly
The cage model (Cube with no vertices Image:Huddy2024-cube-noverts-cage-o4-32.pdb.gz) has about 272,000 atoms including hydrogens. It was simplified to alpha carbon atoms only (17,256) using the Jmol.jar Java application using Jmol commands "select *.ca; write 0.pdb;". The assembled cage has 24 protein chains.
- In Jmol.jar, 4 chains were dragged out of the assembly to the periphery of the viewport, and each was rotated arbitrarily using the Jmol setting "set picking dragmolecule" (holding down Alt enables rotation). The resulting state was written into a PDB file.
- Repeating this drag/rotate save process six times produced a final model in which none of the protein chains were in their original assembled positions. This resulted in six PDB files 1.pdb, ... 6.pdb.
- The linear morph server by Karsten Theis was used on each pair of PDB files to morph in reverse (towards assembly), producing six morphs 6->5, 5->4, ... 1->0 (where 0 is the fully assembled cube).
- Using a [[Help:Plain text editors plain text editor], the morph files were concatenated in the direction of assembly into a single assembly simulation PDB file.
- The junctions between the six original morph PDB files have one duplicate frame. Those duplicates were removed by text editing.
- An empty (no atoms) MODEL 0/ENDMDL was added to the beginning of the PDB file. This blanks the molecular display between cycles of assembly simulation.
- A morph of the assembled cube rotating slightly was made, and added to the end of the PDB file as the conclusion of the assembly simulation.
- After loading the final assembly simulation PDB file into Jmol, the simulation is played with this command script, which is included in the "Play Assembly Simulation" green link above:
reset;center {-10.484505 11.968994 4.189499}; rotate z -0.04; rotate y 90.22; rotate z -0.42; zoom 112.62;
background white
select all
spacefill 3.0
color chain
anim mode loop 0.5 2.0; # not palindrome
anim on;
anim fps 8; # frames per second
The first line in the above script was copied from the report by Jmol after orienting the cube as desired, and entering the command "show orientation".
See Also
- Related work from the Baker group includes Bond-centric modular design of protein assemblies by Wang et al., 2024[9].
- Metal-Ligand Polyhedra
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Huddy TF, Hsia Y, Kibler RD, Xu J, Bethel N, Nagarajan D, Redler R, Leung PJY, Weidle C, Courbet A, Yang EC, Bera AK, Coudray N, Calise SJ, Davila-Hernandez FA, Han HL, Carr KD, Li Z, McHugh R, Reggiano G, Kang A, Sankaran B, Dickinson MS, Coventry B, Brunette TJ, Liu Y, Dauparas J, Borst AJ, Ekiert D, Kollman JM, Bhabha G, Baker D. Blueprinting extendable nanomaterials with standardized protein blocks. Nature. 2024 Mar;627(8005):898-904. PMID:38480887 doi:10.1038/s41586-024-07188-4
- ↑ Brunette et al. (David Baker lab), [https://doi.org/10.1101/2025.01.29.635581 A Multivalent Pan-Ebolavirus Nanoparticle Vaccine Provides Protection in Rodents from Lethal Infection by Adapted Zaire and Sudan Viruses], bioRXiv Preprint, February, 2005.
- ↑ An amino-terminal histidine tag was not resolved in the electron density map of 8g9j, and thus is missing in the structure depicted.
- ↑ de Haas RJ, Brunette N, Goodson A, Dauparas J, Yi SY, Yang EC, Dowling Q, Nguyen H, Kang A, Bera AK, Sankaran B, de Vries R, Baker D, King NP. Rapid and automated design of two-component protein nanomaterials using ProteinMPNN. Proc Natl Acad Sci U S A. 2024 Mar 26;121(13):e2314646121. PMID:38502697 doi:10.1073/pnas.2314646121
- ↑ Sumida KH, Núñez-Franco R, Kalvet I, Pellock SJ, Wicky BIM, Milles LF, Dauparas J, Wang J, Kipnis Y, Jameson N, Kang A, De La Cruz J, Sankaran B, Bera AK, Jiménez-Osés G, Baker D. Improving Protein Expression, Stability, and Function with ProteinMPNN. J Am Chem Soc. 2024 Jan 9. PMID:38194293 doi:10.1021/jacs.3c10941
- ↑ Dauparas J, Anishchenko I, Bennett N, Bai H, Ragotte RJ, Milles LF, Wicky BIM, Courbet A, de Haas RJ, Bethel N, Leung PJY, Huddy TF, Pellock S, Tischer D, Chan F, Koepnick B, Nguyen H, Kang A, Sankaran B, Bera AK, King NP, Baker D. Robust deep learning-based protein sequence design using ProteinMPNN. Science. 2022 Sep 15:eadd2187. doi: 10.1126/science.add2187. PMID:36108050 doi:http://dx.doi.org/10.1126/science.add2187
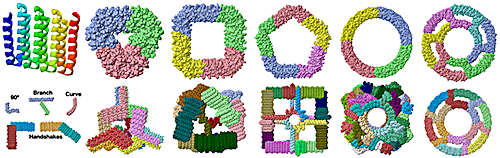
- ↑ The five modules shown are from the following assemblies that can be downloaded as PDB files from supplementary materials of Huddy et al,, 2024: 90° from strut_C6_16. Branch from TT_rail+_tie+. Curve from R20A. Handshake 90° from cage_O4_32. Handshake obtuse angle from cage_I3_8.
- ↑ The assemblies shown can be downloaded as PDB files from supplementary materials of Huddy et al,, 2024.
- ↑ Wang S, Favor A, Kibler R, Lubner J, Borst AJ, Coudray N, Redler RL, Chiang HT, Sheffler W, Hsia Y, Li Z, Ekiert DC, Bhabha G, Pozzo LD, Baker D. Bond-centric modular design of protein assemblies. bioRxiv [Preprint]. 2024 Oct 12:2024.10.11.617872. PMID:39416012 doi:10.1101/2024.10.11.617872