We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 641
From Proteopedia
proteopedia linkproteopedia link
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685.
|
To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
For more help, look at this link:
http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
Glutamate Dehydrogenase
Introduction
- Glutamate Dehydrogenase (GDH)is used to remove the ketone group and replace it with an α-amine group on the α-carbon, which forms glutaamte. Glutamate is one of the 20 essential amino acids. This is done in reverse to supply α-ketoglutarate to the tricarboxylic acid (TCA) cycle. GDH is an oxidoreductase, which is an enzyme that transfers electrons from one molecule (reductant/electron donor) to another molecule (oxidant/electron acceptor).
-
Structure
Glutamate Dehydrogenase is a hexamer that is comprised of two trimer subunits. These two subunits are stacked and composed of three domains. The top of each domain contains a "NAD-binding domain" that has the conserved nucleotide-binding motif. A larger helix-loop-helix structure rises above this and is referred to as an "antenna." This antenna contains approximately 50 amino acids and plays a major role in regulation of the enzyme. (1)
Mechanism
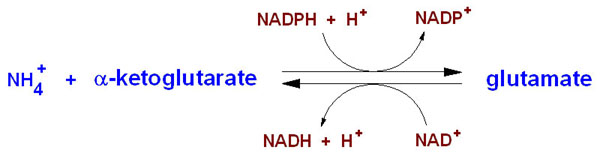
NH4+ + α-ketoglutarate + NADPH + 2 H+ → glutamate + NADP+ + H2O
(1) http://www.sciencedirect.com.prox.lib.ncsu.edu/science/article/pii/S0968000408001898
(2) http://onlinelibrary.wiley.com/doi/10.1111/j.1432-1033.1974.tb03565.x/pdf
(3) http://onlinelibrary.wiley.com/doi/10.1002/prot.340120109/pdf
(4) http://www.sciencedirect.com/science/article/pii/S0969212699801014x/pdf
(3) http://onlinelibrary.wiley.com/doi/10.1002/prot.340120109/pdf
(4) http://www.sciencedirect.com/science/article/pii/S0969212699801014
|

