Sandbox Reserved 648
From Proteopedia
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
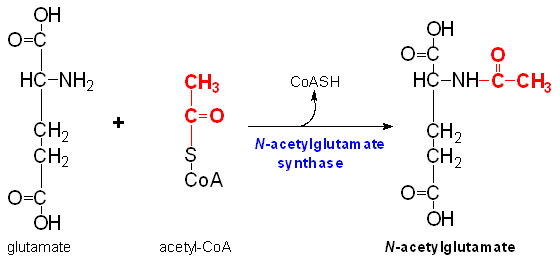
N-Acetylglutamate synthase
IntroductionN-Acetylglutamate synthase (NAGS) is a mitochondrial enzyme involved in the Urea Cycle. This enzyme is most directly used in the conversion of glutamate (glutamic acid) and Coenzyme A into N-Acetylglutamate (NAG). N-Acetylglutamate synthase was first discovered as a mammalian liver enzyme but has very low rate of conservation across phyla. In mammals, N-Acetylglutamate synthase modulates Carbamoyl phosphate synthetase I which is the first rate limiting enzyme in the Urea cycle. Arginine greatly up regulates the activity of NAGS in mammals. Human NAGS is synthesized as a preprotein of 534 amino acids [1]. There is only 63% identity between the mouse NAGS and human NAGS. In bacteria and fungi NAGS is inhibited by arginine.
Structure
Mechanism of actionImplications or possible applicationReferencesFootnotes |