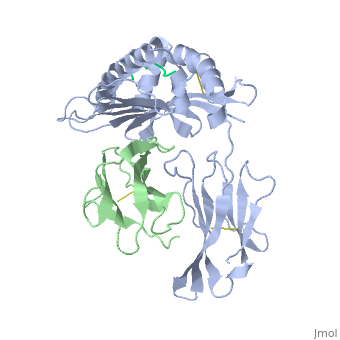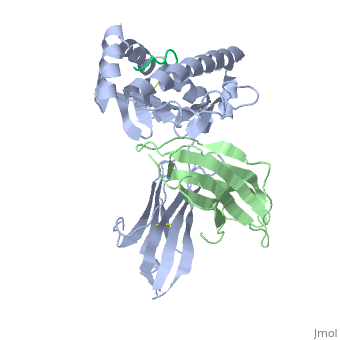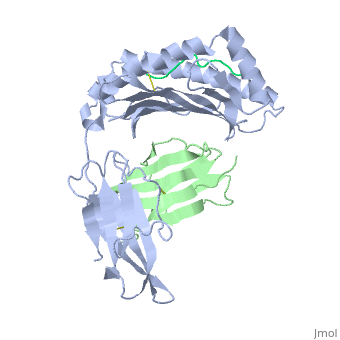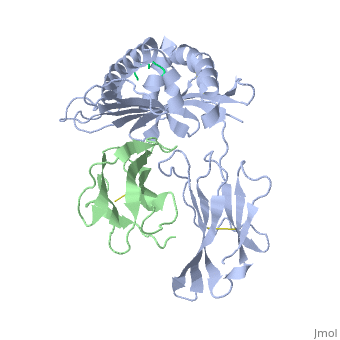
Recovery of properly assembled MR1-β2m complexes indicates efficient capture of a ligand. Using this approach, it was found that MR1 successfully refolded in the presence of folic acid. Further analysis of the complex revealed that 6-formyl pterin (6-FP) was the specific ligand component that allowed proper refolding of MR1. MR1 adopts a standard MHC-I fold. The α1 and α2 domains, which form the antigen-binding cleft, consist of two long α-helices sitting on top of a β-sheet.The binding cleft consists of a mixture of charged and hydrophobic residues.
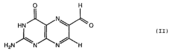
The lies relatively flat against the β-sheet. Binding to 6-FP is dominated by hydrophobic interactions with Tyr7, Tyr62, Trp69 and Trp156. The bulky side chains of these residues form an that sequesters the ligand.
The ligand also formed interactions with Arg9, Arg94, Ile96 and Gln 153.
Adjacent to the antigen-binding cleft, two positively charged residues protrude up into the cleft, suggesting a requirement for polar moieties in other potential MR1-restricted ligands.
A mutational study has shown that are involved in MAIT cell activation.
MR1-restricted MAIT Activation
MAIT cells compose up to 10% of the peripheral blood T-cell population in humans. They are mostly associated with mesenteric lymph nodes and the gastrointestinal mucosa.
[4] They interact with the MR1-antigen complex via their αβ T-cell antigen receptor (TCR). These TCRs consist of an α and β chain, and each chain has a constant (C) and variable (V) domains.
[5] The V domain of each chain has three complimentarity determining regions (CDRs). Together, the six CDRs form a ligand-binding site for the MAIT TCR. The MAIT TCR is restricted to MR1-antigen presentation. Mutational data suggests that a limited number of Vα chain residues of the MAIT TCR are critical for MR1-induced activation.
[6]
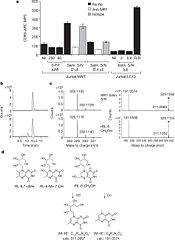
Identification of bacterially-derived MAIT-cell antigens
Even though 6-FP is a ligand for MR1, it does not activate MAIT cells. It was found that antigen-presenting cells transfected with MR1 and infected with Salmonella typhimurium specifically activated MAIT cells. Notably, the supernatent from S. typhimurium was able to activate MAIT cells. Activation was assayed by CD69 upregulation and intracellular cytokine staining for interferon (IFN)-γ and tumor necrosis factor (TFN).[7] By searching for ligands from the supernatent that complexed with MR1, it was revealed that candidate MAIT-activating ligands are derivatives of riboflavin (vitamin B2).[8] These vitamin metabolites are structurally close to 6-FP, but they possess an extra ribityl moiety that may permit direct contact by the MAIT TCR. These compounds are derived from the riboflavin biosynthetic pathway present in most, but not all, bacteria and yeast.
Biological Significance
There are a wide range of microbes that stimulate MAIT cells via MR1 interaction, and they all synthesize riboflavin. This suggests a possible mode by which MAIT cells might sense microbial infection or overgrowth at mucosal sites in an MR1-restricted manner.[9] There are possibly many species of bacteria in the gut flora that have riboflavin biosynthetic pathways. Perhaps the relatively frequent stimulation of MAIT cells serves as way for the body to strengthen the immune system, so as to not be overcome by the co-existing microbiota. The human microbiome is very delicately balanced. It is possible that a spiked growth of an opportunistic pathogen could trigger extensive MAIT cell activation (via MR1) to cause an effective immune response against the pathogen. There also could be other microbial-specific metabolites[10]that may serve as indicators of microbial infections that undergo immunosurveillance.
The pterin ring, an important component of MR1 ligands, occurs widely in nature and also represents a common scaffold of small molecule therapeutics.[11] Further research must be done to determine if MAIT cell-MR1 interactions are affected by drugs and diet.