Sandbox Reserved 771
From Proteopedia
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
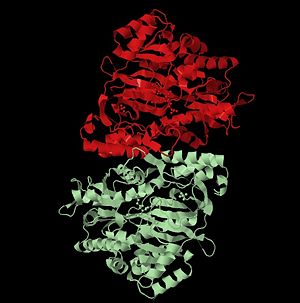
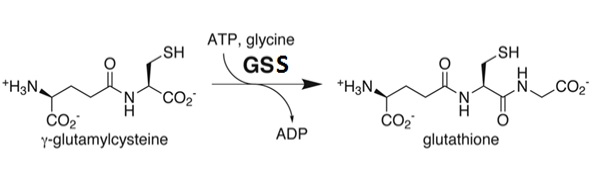
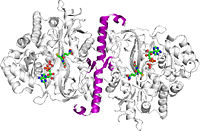
Glutathione synthetase (GSS) is an homo-dimeric, ATP-depending ligase responsible for the condensation of γ-Glutamylcysteine and glycine to form Glutathione (GSH) during the second step of the glutathione biosynthesis pathway [1]. Glutathione is considered to be one of the most abundant and important antioxidants present across many bacterial (cyano- and proteobacteria), and all plant & mammalian cells [2]. In addition to protecting cells from the oxidative damage caused by free radicals, it is believed to be involved in the detoxification of xenobiotics, toxins in the blood, and even amino acid transport [3].
Reaction Mechanism
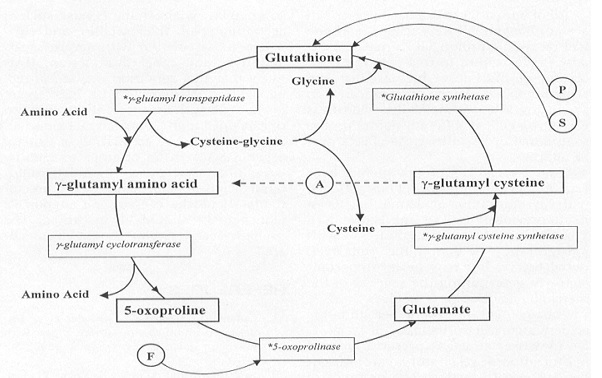
Glutathione Synthetase is the key enzyme involved in the ATP-dependent condensation of γ-Glutamylcysteine and glycine to form Glutathione during the second step of the glutathione biosynthesis pathway [4]. [5]. The condensation begins by binding of ATP to GSS in the presence of γ-Glutamylcysteine, to form an enzyme-bound acyl-phosphate that binds glycine and generates the enzyme-product complex. Dissociation of GSS from the E::P complex results in release of GSH, ADP, and inorganic phosphate (Pi) [6]. The ATP-dependence of the catalysis qualifies GSS for inclusion into the ligase enzyme superfamily. Further, a Hill constant of ~0.67 indicates that GSS exhibits negative cooperativity towards the substrate γ-Glutamylcysteine [7].
The glutathione biosynthesis pathway is an inter-dependent cycle, exhibiting a regulatory ability through the negative cooperativity of the second step of the cycle -- the step catalyzed by GSS [8]. Below, you can see the full cycle including the substrates, cofactors, and enzymes involved in each step of the reaction.
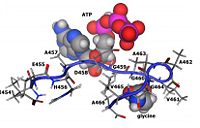
Substrate and ATP Binding Residues
| |||||||||||
References
- ↑ Uchida M, Sugaya M, Kanamaru T, Hisatomi H. Alternative RNA splicing in expression of the glutathione synthetase gene in human cells. Mol Biol Rep. 2010 Apr;37(4):2105-9. doi: 10.1007/s11033-009-9675-3. Epub 2009, Aug 12. PMID:19672693 doi:http://dx.doi.org/10.1007/s11033-009-9675-3
- ↑ http://www.ncbi.nlm.nih.gov/protein/NP_000169.1
- ↑ Slavens KD, Brown TR, Barakat KA, Cundari TR, Anderson ME. Valine 44 and valine 45 of human glutathione synthetase are key for subunit stability and negative cooperativity. Biochem Biophys Res Commun. 2011 Jul 8;410(3):597-601. doi:, 10.1016/j.bbrc.2011.06.034. Epub 2011 Jun 12. PMID:21683691 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.034
- ↑ http://www.ncbi.nlm.nih.gov/protein/NP_000169.1
- ↑ 21771585
- ↑ Dinescu A, Brown TR, Barelier S, Cundari TR, Anderson ME. The role of the glycine triad in human glutathione synthetase. Biochem Biophys Res Commun. 2010 Oct 1;400(4):511-6. doi:, 10.1016/j.bbrc.2010.08.081. Epub 2010 Aug 26. PMID:20800579 doi:http://dx.doi.org/10.1016/j.bbrc.2010.08.081
- ↑ Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. Aspartate 458 of human glutathione synthetase is important for cooperativity and active site structure. Biochem Biophys Res Commun. 2011 Aug 5;411(3):536-42. doi:, 10.1016/j.bbrc.2011.06.166. Epub 2011 Jul 12. PMID:21771585 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.166
- ↑ Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. Aspartate 458 of human glutathione synthetase is important for cooperativity and active site structure. Biochem Biophys Res Commun. 2011 Aug 5;411(3):536-42. doi:, 10.1016/j.bbrc.2011.06.166. Epub 2011 Jul 12. PMID:21771585 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.166
- ↑ Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. Aspartate 458 of human glutathione synthetase is important for cooperativity and active site structure. Biochem Biophys Res Commun. 2011 Aug 5;411(3):536-42. doi:, 10.1016/j.bbrc.2011.06.166. Epub 2011 Jul 12. PMID:21771585 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.166
- ↑ Slavens KD, Brown TR, Barakat KA, Cundari TR, Anderson ME. Valine 44 and valine 45 of human glutathione synthetase are key for subunit stability and negative cooperativity. Biochem Biophys Res Commun. 2011 Jul 8;410(3):597-601. doi:, 10.1016/j.bbrc.2011.06.034. Epub 2011 Jun 12. PMID:21683691 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.034
- ↑ Dinescu A, Brown TR, Barelier S, Cundari TR, Anderson ME. The role of the glycine triad in human glutathione synthetase. Biochem Biophys Res Commun. 2010 Oct 1;400(4):511-6. doi:, 10.1016/j.bbrc.2010.08.081. Epub 2010 Aug 26. PMID:20800579 doi:http://dx.doi.org/10.1016/j.bbrc.2010.08.081
- ↑ Uchida M, Sugaya M, Kanamaru T, Hisatomi H. Alternative RNA splicing in expression of the glutathione synthetase gene in human cells. Mol Biol Rep. 2010 Apr;37(4):2105-9. doi: 10.1007/s11033-009-9675-3. Epub 2009, Aug 12. PMID:19672693 doi:http://dx.doi.org/10.1007/s11033-009-9675-3
- ↑ PMID:10369661</red>. <ref>http://ghr.nlm.nih.gov/condition/glutathione-synthetase-deficiency</li></ol></ref>