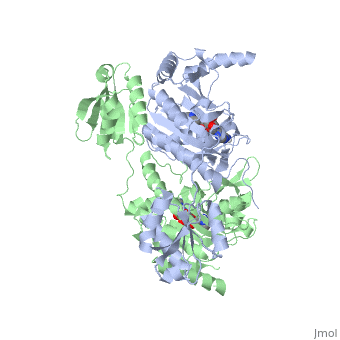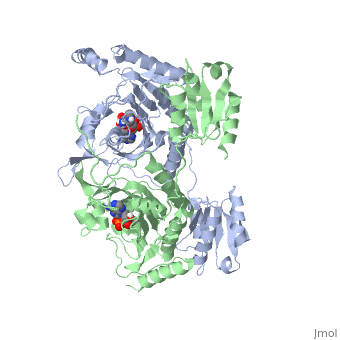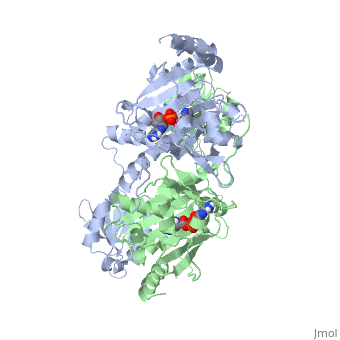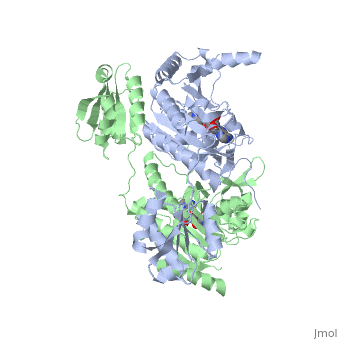
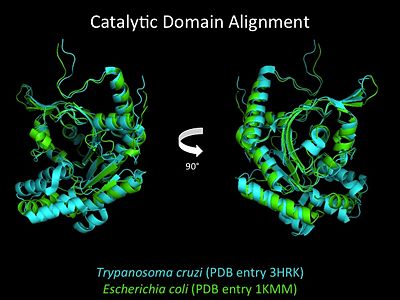
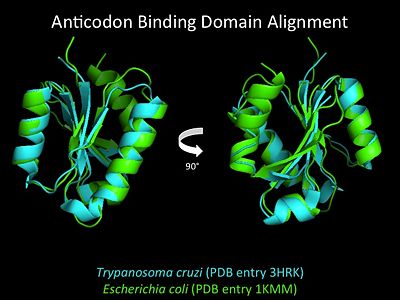
Histidyl tRNA Synthetase (HisRS) is a 94kD that belongs to the class II of aminoacyl-tRNA synthetases (aaRS). Aminoacyl-tRNA synthetases play a key role in protein synthesis and are classified as ligases. Aminoacyl tRNA synthetases use high energy ATP to attach a specific amino acid to its cognate tRNA[1]. This family of enzymes have been partitioned into two classes, containing 10 members, on the basis of sequence comparisons[1]. Class I and class II enzymes differ mainly with respect to the topology of the catalytic fold and site of esterification on cognate tRNA[1]. Class I aaRS enzymes contain a conserved Rossmann fold catalytic domain and often monomeric. Furthermore, class I enzymes attach the activated amino acid to the 2'OH of the tRNA molecule during catalysis, which then migrates to the 3'OH. Class II enzymes have a composed of anti-parallel (residues 1-325). Additionally, class II enzymes can be further divided into three subgroups: class IIa, distinguished by an N-terminal catalytic domain and C-terminal accessory domain (later shown to be the ); class IIb, whose anticodon binding domain is located in the N-terminal region; and class IIc, encompassing the tetrameric PheRS and GlyRS class II synthetases [2].
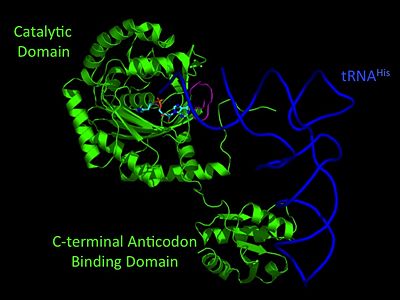
Histidyl-tRNA synthetases catalyze the transfer of histidine to a histidinyl transfer RNA molecule (tRNAHis). As HisRS is a class II aaRS enzyme it attaches the amino acid histidine to the 3’OH of the terminal ribose of tRNA[3]. Histidine is often an important amino acid in the active site of other enzymes and is unique as it can behave as either an acid or a base. The overall secondary structure of a HisRS monomer consist of 20% beta sheets and 37% helical character. It is structurally classified by CATH as an α-β layered sandwich. The CATH hierarchy of structural classification is based on: Class, Architecture, Topology, Homologous superfamily. Each of HisRS contains a N-terminal catalytic domain, C-terminal anticodon binding domain, a , II, and III, an insertion domain, a HisA and HisB loop, as well as an insertion domain. These structural elements are essential in assisting the two step mechanism carried out to aminoacylate tRNAHis with high fidelity.
Substrate Specificity
The first crystal structure solved for histidyl tRNA-synthetase was of E.coli. To date structures of E.coli HisRS complexed with ATP, AMP, histidine, competitive inhibitor (histidinol), histidyl-adenylate, and 5’-O-[(L-histidinylamino)sulfonyl] adenosine have been extensively explored. Many residues involved in substrate specificity and binding were identified. Not only have residues important for substrate binding been identified but also residues essential for catalysis.
Histidine Binding Pocket
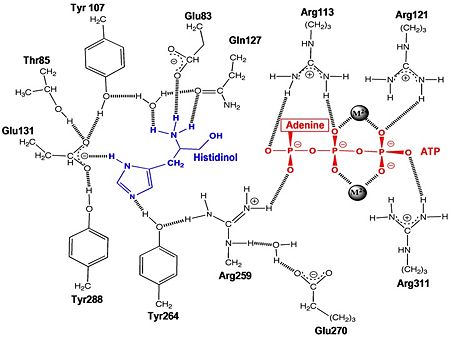
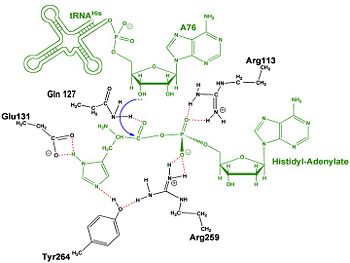
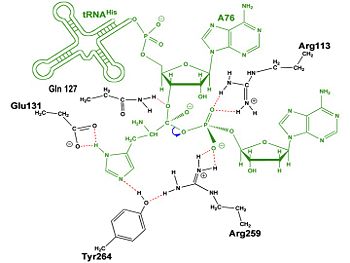
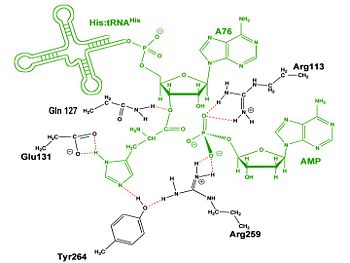
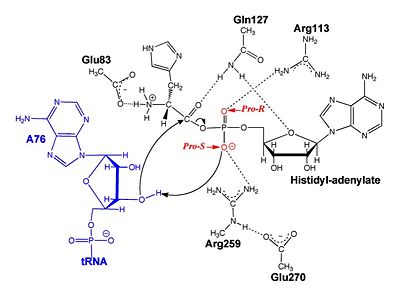
The active site of HisRS contains a composed of highly conserved found in distinct sequences motifs. First, the LV/AAGGGLDYY loop (or ) forms one wall of the binding pocket. This HisA loop is highly conserved and extends over a part of the active site[3]. Second, the glycine-rich β-strand (sequence AGGRYDGL preceding ) comprises the histidine binding pocket floor and wall. Finally, conserved side chains that make direct contact with histidine are Glu83 and Gln127 (), which contact the α-amino and α-carbonyl functional groups, respectively, and Glu131 (motif II) and Tyr264, which make hydrogen bonds to the Nδ and Nε, respectively, of the imidazole ring[3].
ATP Cofactor Binding
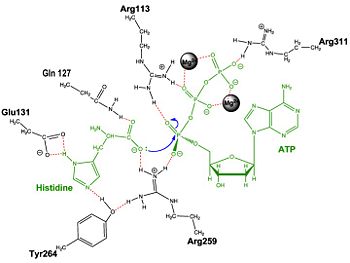
Many interactions are required to prepare adenosine triphosphate (ATP) for attack by a bound histidine molecule and encourage the magnesium pyrophosphate moiety to act as a leaving group. Residues in the β strands and the loop portion of motif 2 are important in ATP contacts for HisRS[4]. Generally, residues involved in are among the most highly conserved in the HisRS family and for the most part shared by all members in class II. The π-stacking interaction between the adenine ring of ATP and provides specificity in the binding of ATP. The recognition of the N6 amino group of ATP involves the main chain carbonyl of Tyr122. The ATP ribose 2’ OH forms an additional contact with HisRS by hydrogen bonding with the main chain carbonyl of Thr281. There are four that mediate interactions with the triphosphate group of ATP. First, the conserved Arg113 forms a bridging interaction with the α and β phosphates of ATP in the crystal structure of e.coli HisRS complexed with histidinol [4]. Also, the γ phosphate forms salt bridges with conserved Arg121 and Arg311 in a complex with ATP. However, when HisRS is complexed with the adenylate, the Arg121 and Arg311 interactions are absent and adopt different conformations[5][4]. Furthermore, Glu115 also assists in the stabilization of the triphosphate group of ATP in a position such that it points back towards the adenine base. This conformation of ATP is evidently unique to class II aaRS[6].
The α phosphate of ATP interacts with conserved residue Arg113. The β and γ phosphates are neutralized by two coordinated magnesium ions that are positioned by water molecules and conserved Glu115[3]. The γ phosphate also forms interactions with conserved Arg121 and Arg311.
A Role for Coordinated Metal Ions
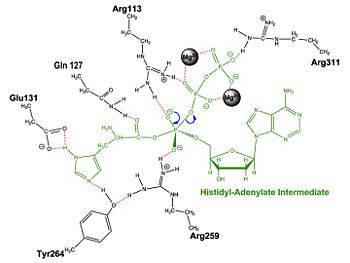
Thus far, residues of HisRS have been described for the binding of substrates histidine and ATP. However, HisRS also requires two magnesium ions to carry out catalysis. Most importantly, the β and γ phosphates of ATP are neutralized by two coordinated magnesium ions that are positioned by water molecules and conserved Glu115. Weak electron density, consistent with a bound Mg
2+ ion, was observed in an electron density map for the HisRS:histidinol:ATP complex
[4]. This particular Mg
2+ ion coordinates the β and γ phosphates of ATP. Arnez et al. further defined the locations of magnesium ions by taking a crystal of HisRS:histidinol:ATP complex and soaking it in manganese(II) chloride MnCl2. These data showed that two Mn
2+ ions coordinate the β and γ phosphates of ATP. Furthermore, interatomic distances between the Mn
2+ principal ion and the β phosphate oxygen is approximately 0.5 Å, which would be expected to contribute to catalysis by weakening the bond between the α and β phosphates of ATP. In similar manganese soaking experiments with another classIIa aaRS, SerRS, the principal metal ion was shown to coordinate the α and β phosphates
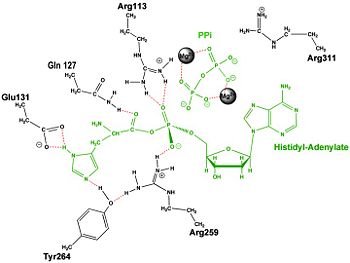
[7]. The functional role for the metal ion coordination between the α and β phosphates for SerRS is a metal-catalyzed mechanism for the adenylation reaction. Interestingly, Arg259 in the HisRS:ATP complex resides in the position occupied by the metal catalyst Mg
2+, in classIIa SerRS. Arg259 and Arg113 serving in place of a Mg
2+ ion is unique to HisRS compared to other classII aaRS. Other classII aaRS enzymes have conserved carboxylate groups to assist coordination of metal ions to carry out catalysis, while HisRS has in place residues Glu270 and Thr281 that have poor geometry for metal coordination but participate in the arginine salt bridge switch
[4].