Function
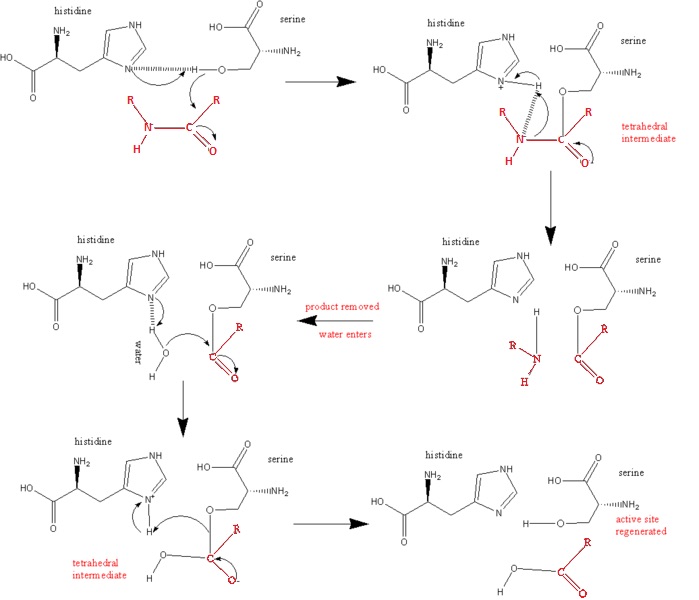
Trypsin is a serine protease released by the pancreas and secreted into the duodenum that acts as a digestive enzyme that catalyzes the hydrolysis of peptide bonds, specifically for positively charged residues (K, R, H). The catalytic mechanism in which the enzyme acts as a protease is as follows:
1. Nucleophillic and base catalysis by enzyme to substrate to form tetrahedral intermediate at carbonyl group of scissile peptide.
The nucleophilic attack is carried out by Ser 195, by attacking the scissile peptide's carbonyl group to form the tetrahedral intermediate.
2. Acid catalysis breaks the tetrahedral intermediate through cleaving of the scissile peptide bond to form an acyl-enzyme intermediate. His 57 donates a proton by general acid catalysis. This is aided by Asp 102 polarizing effect on His 57. This causes the tetrahedral intermediate to decompose to the acyl-enzyme intermediate.
3. The amine product is replaced by H2O and subsequently released from the enzyme/substrate complex.
4. Base catalysis by enzyme; H2O forms a covalent bond with the carbonyl group of the N-terminal peptide, leading to another tetrahedral intermediate
5. Acid catalysis by the breaking of the C-O covalent bond of the tetrahedral intermediate, releasing the peptide from the enzyme substrate complex. Once the peptide is released, the enzyme once again becomes active. [1].
Below is a Diagram of the Catalytic Mechanism:
Structural highlights of Trypsin
Ser 195 nucleophilically attacks the scissile's peptide's carbonyl group
The N3 of His 57 donates a proton (General Acid Catalysis)