|
ISSN 2310-6301
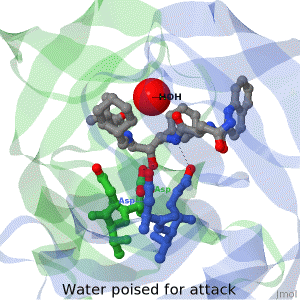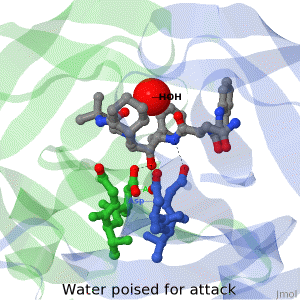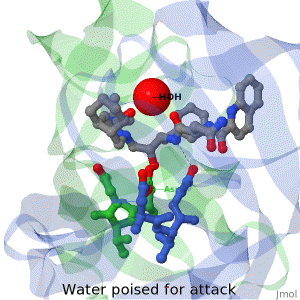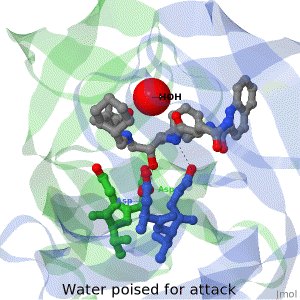
As life is more than 2D, Proteopedia helps to bridge the gap between 3D structure & function of biomacromolecules
|
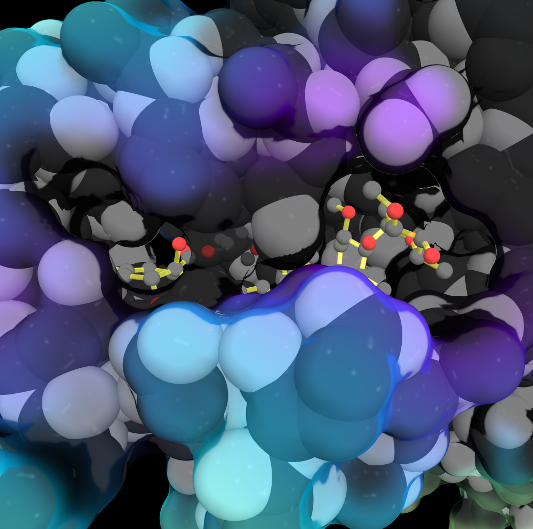
Often it is difficult to utilize the wealth of information found in 3D biomacromolecular structures. Proteopedia's goal is to present structure/function information on these molecules in a user-friendly manner to a broad scientific audience.
</td></tr>
| Selected Pages |
Art on Science |
Journals |
Education |
| |
|
|
|
|
|
|
|
|
How to add content to Proteopedia
Video Guides
Who knows ...
|
List of Art on Science pages
|
About Interactive 3D Complements - I3DCs
List of I3DCs
How to get an I3DC for your paper
|
Teaching strategies using Proteopedia
Examples of pages for teaching
How to add content to Proteopedia
|
|
|