Introduction
Histone methylation
Histone Methylation
Structure
SET Domain
The (HKMT) SET7/9 is 366 amino acids long. The overall structure looks like a dimer although it acts as a monomer. The structure is composed of the ΔSET7/9 domain. The ΔSET7/9 consists of the SET domain along with the pre- and post-SET regions.[1][2] The pre- and post-SET regions are adjacent to SET domain and are cysteine rich.[1][2] The pre-SET cysteine region is located near the N-terminal where the post-SET region is located near the C-terminal of the domain.[1][2] These regions are said to play an important role in substrate recognition and enzymatic activity.[1][2]
The SET domain is mostly defined by with the few .[1] form a β-hairpin that sticks out at a right angle to the surface of the enzyme.[3] The following three residues () accommodate a sharp bend in the peptide chain and the end of the protein adopts an .[3] The two most defining features of the SET domain are the C-terminal tyrosine and the knot-like fold. These two components have been recognized to be essential for (SAM) binding and catalysis.[1] [2] [4] The knot-like fold contains the binding sites for the cofactor SAM and the peptide substrate.[5]
Active Site and Channel
The most notable feature of the HKMT is the presence of the lysine access channel as the active site. The cofactor and peptide substrate are actually located on opposite sides of the SET domain but are connected through this narrow channel.[3] This channel allows these two components to interact and complete the methyltransfer. The active site in general is considerably tyrosine rich. Residues Tyr245, His297, Ser268, Tyr305, Tyr335, and Tyr337 all help to shape the active site and the channel.[3] The cofactor involved, SAM, provides the methyl for methylation of the lysine on its sulfur atom.
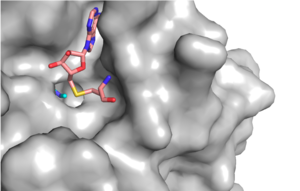
Figure 1: Narrow Lysine Channel
The beta hairpin stabilizes the conformation of Tyr335 and Tyr337, while also shaping one side of the channel which the peptide binds to (Figure 1).[3] The is composed of residues 255-268.[3] Lysine would have trouble coming down into the active site in its charged form, but it is facilitated by the faces of the flanking tyrosines.[3] The orientation of the lysine is such that the amine-methyl bond is aligned towards the sulfur on SAM so that it can provide the methyl. There is an important water in the active site as well that acts as a stabilizer for lysine, and helps to shift the lone pair on the nitrogen towards the sulfur of SAM. [3]
Function
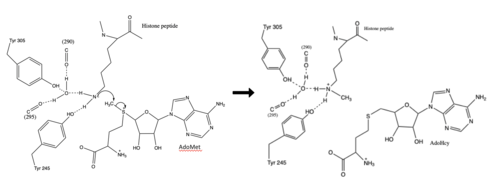
Figure 2: Histone Methylation Mechanism
Relevance
Disease


