Lignostilbene-α,β-dioxygenase A (LsdA) is a catalytic enzyme found in bacteria to break down cellulose components.
Function
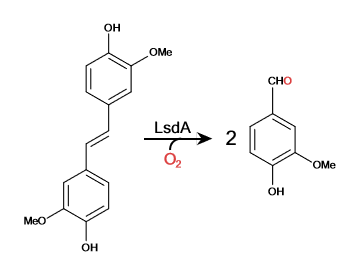
Lignostilbene-α,β-dioxygenase A (LsdA) is an enzyme found in the bacteria Sphingomonas paucimbilis.[1] It is used to catalyze the substrate, lignostilbene, into two vanillin molecules. Vanillin is a member of the benzaldehydes class and plays a role in the plant metabolite[2]. Lignin is found in the plant cell walls, lignocellulose, and it gives the plant its rigid structure. The depolymerization of the lignin can allow for better use of the plants resources as a source of biofuel.
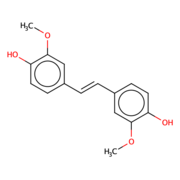
Lignostilbene
The lignostilbene is a compound found in cellulose
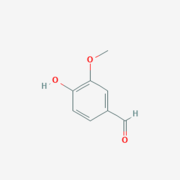
Vanillin
Vanillin is the product from the cleavage of lignostilbene from LsdA.
Cleavage of Lignostilbene
Broader Implications
Lignocellulosic biomasses have been used for biofuels, mainly bio-ethanol. The abundant avaiability of the raw material make the use of biomasses ideal. With the rise of carbon emission, biomasses can be used to substitue the need for carbon based fuel, as long as, the biomass is carbon neutral [3]. Lignostilbene is the second most abundant raw material.
Energy Transformation
When Phe 59 is substituted with His and Tyr 101 is substituted with Phe, the k (app/cat) decreases in activity by 15-10 folds. The Lys 134 substitution of Met inhibits the enzyme activity. Phenylazophenol inhibits the LsdA-catalyzed cleavage of lignostilbene with a competitive and uncompetitive inhibition.
Structural highlights
The
The regions are shown in grey and the polar regions are in magenta.
Lignostilbene is a structure with two identical protein chains working together. The proteins are partially composed of (Blue) and the majority by indicated in red. Together the structure work to form its relationship and interactions within itself and other bio-molecular compounds found in the environment.
A is commonly found in the active site of the protein and binds to the ligand. The triad for LsdA are identified as PHE 59, TYR 101, and LYS 134. The are found within the two chains of the protein structure accompanied by an Iron ion.