Introduction
Vitamin K epoxide reductase (VKOR) is the enzyme responsible for regenerating vitamin K from vitamin K epoxide to support blood coagulation.
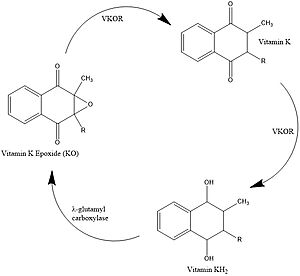
Figure 1. Vitamin K Cycle
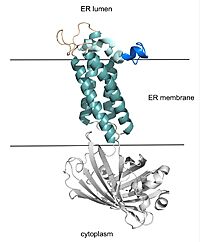
Figure 2. VKOR with Barrel Domain
Vitamin K Cycle
Vitamin K is essential for blood clotting in the body. The fully reduced form, KH2, allows the gamma carboxylation of blood clotting cofactors and is turned into the epoxide form in the process. Vitamin K epoxide reductase turns the epoxide back to the fully reduced form so the reduced form can be used again. This transformation happens in two steps including converting the epoxide to partially oxidized Vitamin K then converting the partially oxidized Vitamin K to the fully reduced hydroquinone. [1]
Structural Overview
VKOR consists of four embedded in the endoplasmic reticulum membrane. The files on the RCSB Protein Data Bank include a barrel domain that is not pertinent to the function of VKOR. The images presented here have been edited to remove the barrel domain and renumbered to correspond with the article by Liu. [2]. The original image with the barrel domain in context is shown in Figure 2. Helices one and two are connected by the beta hairpin region which contains two of the active cysteines, C43 and C51. VKOR also has a cap domain covering the active site, made up of an anchor, loop, and helix.
Function
Catalytic Cycle
Overview
The catalytic cycle shows how vitamin K epoxide reductase structurally transforms from an open wild type conformation to having several different types of substrates within its binding pocket. The first step of the catalytic cycle of shown to the right is the wild type open conformation, labeled I. This step is characterized by an open cap domain with a disulfide bond (43-51) and a second disulfide bond (132-135) in the alpha helices. As shown this step can be characterized as closed when warfarin sits within the binding pocket without the disulfide bonds changing so that the cap domain does not actually close. This step is considered closed because vitamin K would not be able to enter the binding pocket in any of its forms. The second step of the catalytic cycle is a closed conformation labeled II. This step is characterized by a disulfide bond between the cap domain and alpha helices, with both containing an SH group. Warfarin can sit within this structure without disrupting and of these sulfur groups. The next step of the cycle, labeled III, is slightly different because KOH or KH (depending on the step of the vitamin K cycle) binds to the cysteine 135 within the alpha helices. This is also a closed structure. Lastly, structure IV of the catalytic cycle is also a closed structure. The major difference is the orientation of the disulfide and cysteine interactions.
Catalytic Cysteines
There are 4 catalytic cysteines that are important to VKOR, 43, 51, 132, and 135. To explain how this works, it is easiest to start with the second state. In the second state, an oxidized or partially oxidized Vitamin K has entered the active site. The stabilizing 51-132 disulfide bond is shown. Then in the third state, 43 has attacked the disulfide bond and made its own bond with 51. You can see 132 has an oxygen. That is because the researchers made a mutation from S to O to force the reaction to stop at that step so the structure could be deduced. In the natural VKOR, that would be a sulfur. The next state, the open state, results from 132 forming a bridge with 135. This allows release of the reduced or partially reduced Vitamin K. All of this disulfide rearranging was working to reduce the Vitamin K, particularly in the 135 position. If we go back to State 2, when Vitamin K first binds, you can see that 135 is not tied up in a disulfide bond. It is available to help the Vitamin K bond. So, it makes sense that once 135 gets forced to bond, the now reduced Vitamin K is released. State 5 is interesting because the disulfide bonds are similar to the open state, but warfarin is actually bound. This represents the binding of warfarin to the fully oxidized VKOR at the end of its cycle. Going back to State 1, the researchers used a mutation at 43 to mimic VKOR’s partially oxidized state. Warfarin can also bind to this state and notice that the disulfide bonds are the same as State 2. Also it is worth pointing out how the disulfide bonds contribute to conformational changes and are affected by conformational changes, which affects their proximity to each other and the active site.
Catalytic Amino Acids
VKOR uses two catalytic amino acids, tyrosine 139 and asparagine 80, to stabilize in all forms and , such as warfarin, in the binding pocket. Tyr139 and Asn80 hydrogen bond to carbonyl groups on both structures and stabilizes them within the binding pocket.
Hydrophobic Interactions
Other than the two previously mentioned hydrogen bonds (Tyr139 and Asn80), vitamin K and antagonists are bound in via hydrophobic interactions within the binding pocket of VKOR. Hydrophobic residues of VKOR such as Phe80, Phe87, and Tyr88, form a hydrophobic tunnel within the binding pocket.
Medical Relevance
Warfarin
Warfarin is a structural mimic of Vitamin K that is used clinically as an anticoagulant.
Superwarfarins
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.


