Background
Anaplastic Lymphoma Kinase (ALK) is a transmembrane receptor and a member of the family of Receptor Tyrosine Kinases (RTKs), a family of biomolecules that are primarily responsible for biosignaling pathways such as the insulin signaling pathway. It was discovered as a novel tyrosine phosphoprotein in 1994 in an analysis of Anaplastic Large-Cell Lymphoma, the protein's namesake. A full analysis and characterization of ALK was completed in 1997, properly identifying it as a RTK, and linking it closely to Leukocyte Tyrosine Kinase (LTK).
Structure
As previously mentioned, ALK is a close homolog of LTK, and together these two homologous proteins create a subgroup within the superfamily of
insulin receptors (IR). ALK is composed of 1620 amino acids in total, with three primary domains. ALK is described as showing the "classic structural features of RTKs, with an extracellular ligand-binding domain, a transmembrane-spanning region, and an intracellular kinase domain." As displayed in Figure 1,
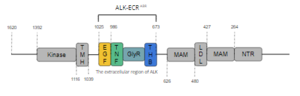
Figure 1. Overview of Anaplastic Lymphoma Kinase Structure
the intracellular tyrosine kinase domain ranges from residues 1116-1392, and features the
C-terminal end. The
transmembrane helical domain (TMH) bridges the gap between the intracellular and extracellular regions from residues 1039-1116. The final section of ALK is the extracellular region, which spans from residues 1025 to 1, and contains 8 domains. Of these 8 domains, two regions of the extracellular region can be found; one containing the ligand-binding site of the protein, and another lesser-known subregion. This lesser-known subregion contains 4 domains from residues 1-626; an
N-terminal Region (NTR), two
meprin–A-5 protein–receptor protein tyrosine phosphatase μ (MAM), and a
low-density lipoprotein receptor class A (LDL) domain sandwiched between the two MAM domains. The presence of an LDL domain sandwiched by two MAM domains is a unique feature that ALK does not share with other RTKs. The purpose behind this unique difference is still unclear. The ligand-binding extracellular subregion is the most well-characterized of the two subregions, containing 4 distinct domains from residues 673-1025; a triple helix bundle (THB) domain, a glycine-rich domain (GlyR) that is also referred to as the poly-Glycine domain, a tumor necrosis factor-like (TNF-like), and an epidermal growth factor-like domain (EGF-like). All four domains of this subregion of the extracellular region contribute to ligand-binding, (more info here).
Domains
Three Helix Bundle-like Domain
The mainly has a structural function overall as it interacts with the tumor necrosis factor-like domain upon ligand binding.
Poly-Glycine Domain
Figure 2. Rare Glycine helices on Anaplastic Lymphoma Kinase
Located between the three helix bundle-like domain and the tumor necrosis factor-like domain, the has an important structural role. The poly-Glycine domain also has a rare and unique structure with hexagonal hydrogen bonding shown in Figure 2.
Tumor Necrosis Factor-like Domain
The interacts with the three helix bundle-like domain to begin the conformational changes associated with ligand binding. The three helix bundle-like domain's α-helix interacts with the helix α-1' and β strand A-1'. This domain also assists in mediating ligand binding with the epidermal growth factor-like domain.
Epidermal Growth Factor-like Domain
The is very malleable and repositioning of this domain is essential for activation of the protein. This domain is able to undergo conformational changes with the ligand bound and when in contact with the tumor necrosis factor-like domain.
Binding Site
This site doesn't start out surrounding the ligand, instead the proximity of the ligand allows conformational changes across the protein. The ligands for ALK both have highly positively charged faces that interact with the TNF-like region[1]. Salt bridges between the positively charged residues on the ligand and negatively charged residues on the receptor form are formed as the ligand approaches connecting the ligand with the receptor.
Ligands
ALKAL2
ALKAL2 (Anaplastic Lymphoma Kinase Ligand 2) is a ligand of ALK as well as LTK located in the extracellular region. The full-length ALKAL2 (dimeric) and ALKAL2-AD (monomeric) can both induce dimerization of ALK [2]. Structurally, ALKAL2 has a N-termical variable region and a conserved augmentor domain and tends to aggregate in the cell [2]. Overexpression of ALKAL2 has been linked to high-risk neuroblastoma in absence of an ALK mutation [3] and could potentially have therapeutic opportunities.
ALKAL1
ALKAL1 (Anaplastic Lymphoma Kinase Ligand 1) is a monomeric ligand of ALK, in addition to ALKAL2. Structurally, ALKAL1 and ALKAL2 contain an N-terminal variable region and a conversed C-terminal augmentor domain [2]. However, in ALKAL1, this N-terminal variable region is shorter, and shares no similar sequences to ALKAL2. Nevertheless, ALKAL1 shares a 91% sequence similarity with ALKAL2. Both ligands include a three helix bundle domain in their structures, with an extended positively charged surface which is used in ligand binding [2].
Dimerization of Anaplastic Lymphoma Kinase
After binding to one of its ligands, Anaplastic Lymphoma Kinase undergoes [4]. The dimerization causes trans-phosphorylation of specific tyrosine residues which in turn amplifies the signal. It has been presumed that the phosphorylation cascade activates ALK kinase activity [4].
Function
Anaplastic Lymphoma Kinase plays a role in cellular communication and in the normal development and function of the nervous system.
Disease and Medical Relevance
Cancer
In ALK fusion proteins, the ALK fusion partner may cause dimerization independent of ligand binding, causing oncogenic ALK activation [4].
The regulation of ALK dimerization by ALKAL points to clear ways to inhibit ALK activity and may offer new therapeutic strategies in multiple disease settings [1]
Neuroblastoma


