Cystathionine gamma-lyase (CSE) is a member of the gamma family of PLP-dependent enzymes (which bind to pyridoxal-5'-phosphate for reaction catalysis), along with other enzymes such as cystathionine gamma-synthase, Cystathionine beta-lyase, and Methionine gamma-lyase. Similar to other enzymes in this family, CSE exhibits a tetrameric structure with D2 symmetry.
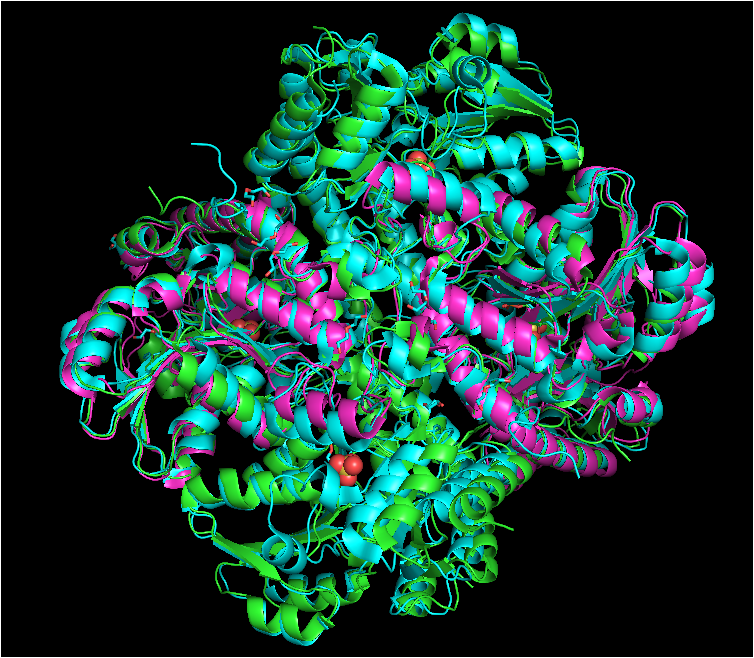
Structure of cystathionine gamma-lyase,
cystathionine beta-lyase, and
methionine gamma-lyase proteins aligned. Cystathionine gamma-lyase is shown in green, cystathionine beta-lyase in purple, and methionine gamma-lyase in blue. Structures aligned vy WinCoot and image generated by PyMOL.
Significant structural overlap can be observed among the majority of alpha helices and beta sheets, with only a limited number of regions exhibiting distinct conformational variations among the proteins.
Functions
Cystathionine gamma-lyase serves two primary functions in Homo sapiens: one is associated with the conversion of L,L-cystathionine into L-cysteine, while the other is linked to the synthesis of H2S (hydrogen sulfide), a signaling molecule implicated in neurological and vascular processes [1].
CSE operates in conjunction with cystathionine beta-synthase (CBS) to facilitate the reverse transsulfuration required for the metabolic interconversion of sulfur-containing amino acids, such as cysteine. Remarkably, reverse transsulfuration is a process exclusive to fungi and mammals. In this intricate process, CBS enzymatically catalyzes the formation of cystathionine from the precursor molecules, homocysteine and serine. Subsequently, CSE mediates the conversion of the synthesized cystathionine into cysteine, alpha-ketobutyrate, and ammonia [2].
CSE also assumes the responsibility for the synthesis of H2S in mammals. This production holds significant importance due to the pivotal role of this compound as a gaseous messenger or gasotransmitter, intricately linked to the functions of the nervous and vascular systems, as well as inflammation. Specifically, CSE is associated with the production of this gasotransmitter in extraneural contexts, whereas CBS fulfills this role within the central nervous system [3]. The enzyme facilitates the formation of H2S through a series of reactions classified into two main categories: cysteine-dependent beta reactions and homocysteine-dependent gamma reactions [1].
Disease
The reduction in cystathionine gamma-lyase activity is associated with a condition known as cystathioninuria, which is characterized by an abnormal accumulation of cystathionine in the bloodstream, leading to an increased excretion of this molecule in the urine[4]. Although mental development disorder has been associated with the condition, it is considered pathologically irrelevant[4]. Cystathioninuria can be classified into primary and secondary forms. The primary form is caused by a genetically inherited deficiency of the enzyme, while the secondary form occurs when the excess cystathionine is not of genetic origin[5].
The genetic inheritance pattern of primary cystathioninuria is autosomal recessive, and multiple mutations have been identified as being associated with the phenotypic expression. Nonsense mutations have been observed in exons 8 and 11 of the gamma-cystathionase gene, whereas missense mutations are primarily located in exons 2 and 7. Furthermore, a non-synonymous single nucleotide mutation in exon 12 has also been reported, resulting in a guanine-to-thymine substitution that leads to the replacement of serine residue 403 with isoleucine in the resultant protein. The concentration of cystathionine in both plasma and urine can be used to distinguish between heterozygous and homozygous individuals[6].
Conservation
The cystathionine gamma-lyase enzymes from multiple organisms form an evolutionarily conserved group of PLP-dependent enzymes, specifically categorized within the cystathionine synthase-like enzyme family. The data exhibits a substantial level of sequence homology among these CSE enzymes at the genomic level. Furthermore, a predominant structural similarity is observed between the active site region of the CSE enzymes and other PLP-dependent enzymes[3].

Alignment of the amino acid sequences of cystathionine gamma-lyase protein from Homo sapiens, Mus musculus, Pseudomonas aeruginosa (
7ba4), and Saccharomyces cerevisiae (
1n8p), showing high similarity across different lineages. Alignment by COBALT NCBI tool.
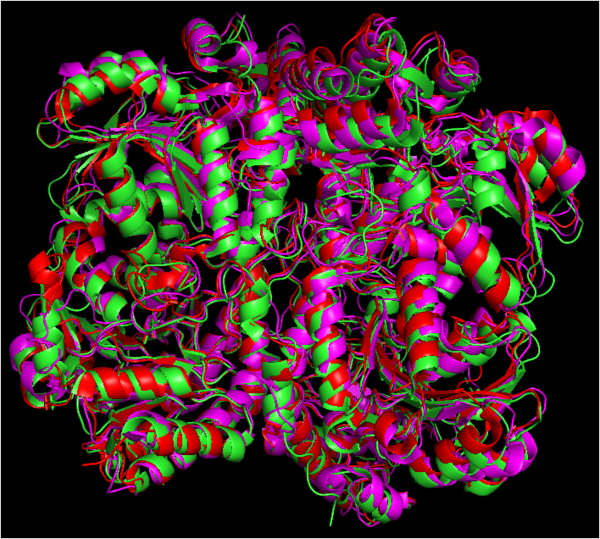
Alignment of the structures of cystathionine gamma-lyase protein from Homo sapiens (green), Pseudomonas aeruginosa (red) and Saccharomyces cerevisiae (purple). Structures aligned vy WinCoot and image generated by PyMOL.
Structure
Cystathionine gamma-lyase exhibits a tetrameric quaternary structure when bound to its ligand (PLP), and in its apo form, it can exist as both a monomer and a tetramer, with the latter being more predominant [3]. The monomer comprises two domains: a .
The is characterized by the presence of an alpha/beta/alpha structure, and a mixed beta-sheet surrounded by eight alpha-helices. On the other hand, the possesses a four-stranded antiparallel beta-sheet and three alpha-helices on one side of the beta-sheet.
Within the larger domain, more specifically in the active for PLP, the amino acid residues that effectively engage with this ligand are situated in the central region of the beta sheet and in close proximity to adjacent alpha helices. A total of six amino acids establish hydrogen bond interactions (Gly90, Leu91, Asp187, Ser209, Thr211, and Lys212). Moreover, two amino acids from the neighboring subunit also establish hydrogen bond interactions with PLP(Tyr60 and Arg62)[3].Another significant aspect is that the amino acid Tyr114 appears to hold great relevance in enzymatic activity, as it interacts with the pyrimidine ring of PLP through aromatic stacking.
Upon juxtaposing the structure of cystathionine gamma-lyase in the absence of the cofactor and its bound state, notable distinctions in the configuration of its active site come to light. In the unbound state, the active site displays a discernibly more unobstructed conformation, whereas its association with PLP induces a marked closure. The most pronounced alterations manifest within (amino acids Met110-Asn118 and Thr210-Met216)[3]. A noteworthy characteristic regarding the conformational changes of this protein is that, in its PLP-bound state, the tetrameric asymmetric unit encompasses one of its subunits lacking the presence of the cofactor (with only 3 subunits remaining bound). This observation suggests that the enzyme exhibits substantial dynamism and is likely to exist as an amalgamation of various conformational states[3].
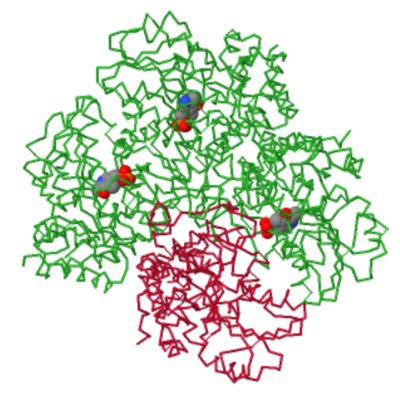
The three subunits that bind to PLP are depicted in green, while the empty unit is shown in red. Image generatade with JSmol.
Another noteworthy observation is that two variants of the CSE protein, associated with cystathionuria, are distinguished by a diminished enzymatic activity owing to a deficiency in PLP binding to CSE. Interestingly, the mutated amino acids in these variants do not encompass the aforementioned residues that engage in direct interaction with the cofactor, thus underscoring the uncertainties and the imperative for additional investigations concerning cystathionine gamma-lyase.
During its enzymatic function, the PLP-bound CSE protein interacts with L-cysteine, leading to the dissociation of PLP from the protein and its subsequent interaction with L-cysteine, resulting in the formation of PLP-iminopropionate and the release of H2S. This interaction is facilitated by the deprotonation of the substrate, catalyzed by the from an adjacent monomer[3], specifically one of the neighboring subunits within the tetramer. This underscores the critical role of the quaternary structure in enzymatic activity of this protein. Another noteworthy aspect of the binding of L-cysteine to CSE pertains to the , which undergoes significant perturbation upon the binding of PLP to the protein. It is hypothesized that this particular amino acid plays a pivotal role in the subsequent release of the substrate following the enzymatic reactions involving the binding of L-cysteine to PLP[3].




