From Proteopedia
proteopedia linkproteopedia link Bromodomain of PfBDP1
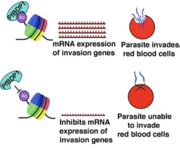
In 2023, globally, there were an estimated 263 million new malaria cases and 597,000 deaths. Plasmodium falciparum, the causative parasite of malaria, invades red blood cells and consumes hemoglobin, preventing oxygen transport to the heart, resulting in heart failure. Additionally, parasitized RBCs stick to the wall of blood vessels in the heart and brain to evade the immune system, which leads to inflammation and blood vessel blockage to these vital organs thus, it is imperative to understand the essential factors involved in the P. falciparum RBC invasion process to develop therapeutic interventions. Previous research has shown that the P. falciparum Bromodomain Protein 1 (PfBDP1) plays an important role in red blood cell invasion by binding to acetylated chromatin at the promoters of invasion genes, promoting their expression. Additionally, knockdown of PfBPD1 impairs Plasmodium falciparum replication and strongly reduces expression of surface proteins involved in red blood cell invasion. This suggests that the chromatin binding activity of PfBDP1 plays an important role in P.falciparum biological fitness. A emerging therapeutic strategy is to block the binding of PfBDP1 to chromatin in order to inhibit the expression of genes implicated in red blood cell invasion.
. The region within the PfBDP1 protein that is involved in chromatin binding, and thus downstream expression of genes that cause malaria, is the bromodomain. Bromodomains are a conserved structural motif within proteins whose primary function is to bind acetylated lysines on histones. Lysine acetylation is a well documented post translational modification on histones that is associated with active transcription. Bromodomains recognize these modifications and recruit transcriptional machinery to active promoters thus eliciting a key regulatory role in gene expression
. The bromodomain of PfBDP1 harbors , which is a conserved structural element amongst bromodomains. The core four helix bundle is arranged in two halves connected by The first half of the four helical bundle is comprised of the connected by the ZA loop, which has a well established role in ligand coordination. The end of the αZ helix encodes a that forms the bottom of the binding pocket, and the long and variable ZA loop (358–378 aa) frames one side of the binding pocket before connecting to the αA helix which is a conserved structural feature of the bromodomain binding pocket. The second half of the conserved bromodomain fold is comprised of the that form the other side of the binding pocket. The αB helix and the αC helix are connected by the 3-residue long BC loop, which contains the conserved asparagine residue that is involved in the coordination of the acetyllysine moiety. Due to bromodomains crucial role in regulating transcription they have been the focus for small molecule inhibitor development. Currently, bromodomains are being targeted to combat cancer and autoimmune diseases however, targeting bromodomains to combat parasite related illnesses remain understudied. Aman et.al utilized virtual docking, isothermal titration calorimetry, and X-ray crystallography to determine the structure of the PfBDP1 bromodomain bound to a derivative of the bromodomain inhibitor MPM6. The involved in coordinating interactions with RMM2 are His354, Ile355, Gln365, Cys367, Asn413, Val419
|
Function
Binds acetylated lysines at promoters of genes implicated in host cell invasion
Disease
Relevance
Structural highlights
|
References