This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
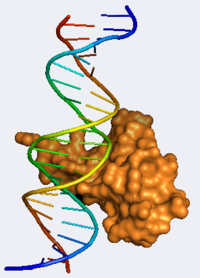
Methyl CpG Binding Protein 2
From Proteopedia
| |||||||||||
Contents |
Page Development
This article was developed based on lectures given in Chemistry 543 by Prof. Clarence E. Schutt at Princeton University.
3D structures of MeCP2
See Methyl CpG binding protein.
See Also
- Introduction to Evolutionary Conservation which uses MeCP2 as an example.
- Brief introduction to Rett Syndrome
References
- ↑ 1.0 1.1 1.2 1.3 Ho KL, McNae IW, Schmiedeberg L, Klose RJ, Bird AP, Walkinshaw MD. MeCP2 binding to DNA depends upon hydration at methyl-CpG. Mol Cell. 2008 Feb 29;29(4):525-31. PMID:18313390 doi:10.1016/j.molcel.2007.12.028
- ↑ Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003 Oct 31;302(5646):826-30. PMID:14593168 doi:10.1126/science.1089071
- ↑ Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010 Nov 12;143(4):527-39. PMID:21074045 doi:10.1016/j.cell.2010.10.016
- ↑ Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007 Feb 23;315(5815):1143-7. Epub 2007 Feb 8. PMID:17289941 doi:10.1126/science.1138389
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Eric Martz, Jaime Prilusky, Alexander Berchansky