Sandbox Reserved 596
From Proteopedia
| This Sandbox is Reserved from Feb 1, 2013, through May 10, 2013 for use in the course "Biochemistry" taught by Irma Santoro at the Reinhardt University. This reservation includes Sandbox Reserved 591 through Sandbox Reserved 599. |
To get started:
More help: Help:Editing |
Contents |
Protein Z
Background
The human body maintains the many aspects of homeostasis, or biological equilibrium, through multiple physiological pathways. Hemostatic mechanisms have evolved over 400 million years to protect against the ever-present danger of fatal hemorrhage. [1] When inflicted by a wound resulting in internal or external bleeding, one of the two converging coagulation cascade branches is triggered to maintain hemostasis. If the wound is just an epithelial surface wound, the body reacts with the intrinsic pathway but if the wound is more traumatic, resulting in more extensive vascular and tissue damage, the extrinsic branch is used. This coagulation pathway is known as a cascade because the product of the previous reaction acts as an enzyme for the following reaction, a flow that can be attributed to the specificity of each enzyme along with positive and negative feedback loops to further control the directionality. [2]
The coagulation cascade involves many coagulation factors that act as serine proteases with active sites centered around their serine residues. These serine protease coagulation factors, like factors VII, IX, X, and protein C are vitamin K-dependent proteins, meaning their γ-carboxyglutamic acid residue (Gla) construction within the liver requires fat-soluble vitamin k. Factor Xa (FXa), an essential enzyme found at the intersection of the two coagulation pathways, is formed on platelet membranes through the interaction of factor IXa, complexed with its cofactor VIIIa, on factor X. FXa complexed with its cofactor Va promotes thrombin production from the cleaved zymogen prothrombin. The cascade continues to flow towards its final processes of prothrombin being cleaved into thrombin and thrombin then cleaving fibrinogen to form a meshwork of fibrin until a blood clot stops the bleeding. To ensure that a clot does not form anywhere other than the injury site, any FXa that dissociates from the membrane must be inhibited. FXa is regulated by two serine protease inhibitors (serpins), antithrombin and protein z-dependent inhibitor (ZPI) that are inactive to FXa until paired with their cofactors heparin and protein z (PZ) respectively. [3]
PZ
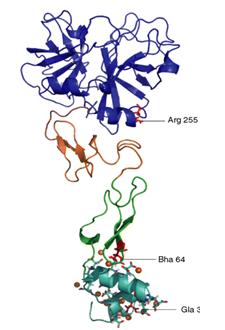
Figure 1
Bovine PZ was first identified by Prowse and Esnouf in 1977 and human PZ, consisting of 360 amino acid residues and having a molecular weight of 62kDa, was first isolated and studied by Broze Jr. and Miletich in 1984. The gene coding for human PZ, PROZ, was found in 1998 on chromosome 13 at location 13q34 and is composed of a 389 bp promoter and nine exons (one being an alternative exon). [1,4] PZ is a single-chain glycoprotein with a 13 γ-carboxyglutamic (residues 7, 8, 11, 15, 17, 20, 21, 26, 27, 30, 33, 35 and 40) acid residues (Gla) N-terminal domain (residues 1-46), two epithelial growth factor (EGF)-like domains (EGF1 residues 47-830, EGF2 residues 85-126), and a C-terminal serine protease (SP)- like domain (residues 135-360) (Figure 1).
PZ is very similar to the factor that it serves to inhibit, FXa. Both are vitamin k-dependent proteins with their EGF2 domain closely grouped with their SP domain. Although 33% homologous in sequence to serine protease factor Xa (FXa), PZ is not enzymatically active because it lacks the serine (Ser195) and histidine (His57) of the catalytic triad, giving its oxyanion hole and S1 pocket an inactive configuration. [5] PZ cannot perform the same interactions as FXa because PZ’s oxyanion hole does not have the same stabilizing interactions. PZ’s activation peptide is 5 residues shorter than FXa, and has a methionine (M312) at position 194 with its side chain pointing in the opposite direction of D194 in FXa. The S1 pocket of PZ is occupied by a glutamine (Q334) at position 216, a large tryptophan (W311) at 193 (G216 and G193 in FXa), and a half turn of 310 helix of residues 189-192 (residues 307-310 in PZ). [4]
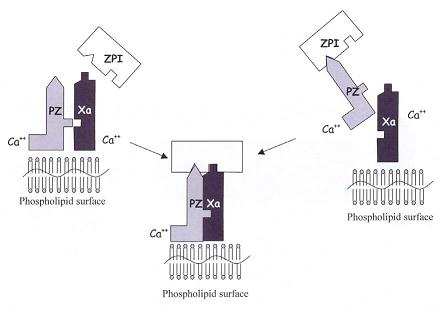
The function of PZ was studied in 1991 by Hogg and Stenflo, who initially hypothesized PZ to amplify the coagulation cascade by interacting with the serine protease thrombin but found that bovine PZ has a higher affinity for thrombin than human PZ due to a 36 amino acid addition to the bovine PZ's C-terminus. Furthermore, they found that human PZ virtually had no involvement in binding thrombin to phospholipids. It was not until 1998 that Han et al. described PZ present in the body as a complex with the serpin protein Z-dependent protease inhibitor (ZPI). The PZ binds to ZPI and then carries the serpin to associate with phospholipid membrane bound FXa (follows right side of Figure 2). [6]
ZPI
|
ZPI, a single-chain glycoprotein made in the liver with 423 residues and a molecular weight of 72 kDa, was first isolated in human plasma in 1998. ZPI is coded for by the gene SERPINA10 at locus 14q32.13 and is 25-35% homologous in its amino acid sequence to the serpin family of protease inhibitors.[7] Structurally ZPI has a characteristic serpin fold with three main β-sheets and exposed reactive center loop (Figure 3- select following link) [[1]]. The ZPI binding site for PZ is centered around its helix G (hG) and helix A (hA) while PZ’s bind site for ZPI is centered around its SP anion-binding site near the C-terminal. ZPI also has an atypical hydrogen bond configuration at the shutter region with the Asn186 of usual serpins being replaced by an aspartate (D213) in ZPI. This causes a different pattern of hydrogen bonds and the resulting placement of the D213 into a hydrophobic area underneath ZPI’s β-sheet A. D213 can therefore only form 1 hydrogen bond, compared to other serpins like antitrypsin and PAI-1 that form 4 and 3 hydrogen bonds respectively. [4]
PZ-ZPI Complex FXa Inhibition
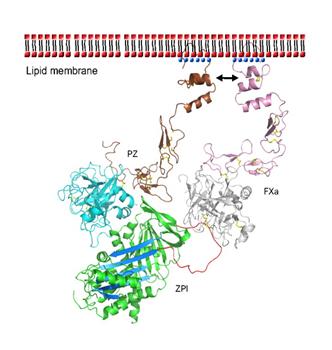
Once PZ has successfully formed a complex with ZPI, it guides the ZPI to activated FXa bound to platelet membranes. The PZ docks itself to the phospholipid membrane through its Gla domain and forms stabilizing bonds with FXa’s similarly membrane-bound Gla domain (Figure 4).
ZPI interacts with FXa’s autolysis loop (E36, E37, or E39) through its negatively charged area (E231, D233, D313) on its top area, which unusually has a positively charged area as well (K253, K260, K308, R310). [4] The resulting PZ-ZPI-FXa tertiary complex is calcium and phospholipid dependent, increasing the rate of ZPI inhibition of FXa by 1000x. After the formation of the calcium-dependent PZ-ZPI-FXa complex and inhibitory changes begin, the PZ dissociates into the bloodstream to be used again. ZPI’s inhibitory action involves being proteolytically cleaved at its C-terminus by FXa, reducing ZPI’s molecular weight from 72 kDa to 68 kDa. [7]
Clinical Relevence
Although PZ is conserved across many different species [5], the complete pathophysiological importance of PZ is still not completely known because many any clinical studies have resulted in conflicting conclusions. In 2001 Rice et al. wished to study just four important polymorphisms of the PROZ gene possibly linked to venous thrombosis but ultimately found and studied that PZ has 14 unique polymorphisms possible due to specific mutations. Lichy et al. studied specific the affects of mutations of Fg79a and promoter A-13g on cerebral ischema.
In 1995 Kemkes-Matthes and Matthes studied PZ’s role in hemorrhagic tendency in patients with low PZ levels, suggesting that PZ deficiency causes the disorder but other groups, Gamba et al. and Ravi et al., researching the same hypothesis did not conclude the same results in their study.
In 2001 Kolbelt et al. studied ischemic cerebrovascular disease (a thrombotic disorder) and found that chronic stroke victims showed high plasma concentrations of PZ but just a few months later Vasse et al. found that ischemic stroke patients had low levels of PZ. Shortly after that in 2002 Lopaciuk et al. found no correlation between PZ levels and chronic stroke and Heeb et al. found low levels associated with stroke but not in women and diabetics.
In 2002 Gris et al. examined PZ levels in pathologic pregnancies and found that low PZ levels caused extreme insufficiency of the placenta following the mother/fetus circulatory connection. Low PZ levels were also linked to antiphospholipid antibodies syndrome (aPL), suggesting PZ-ZPI complex incompetency by Steffano et al. and confirmed by a later study by McColl et al. Kemkes-Matthes et al. found that patients with thromboembolic episodes and factor V Leiden mutations suffered greatly from early thrombosis due to PZ deficiency. Lower levels of plasma PZ have also been linked to chronic inflammatory diseases like Bençet’s disease and ischemic colitis, and acute coronary syndromes (ACS). [1,6]
References
[1] Corral, Javier et al. Protein Z/Z-dependent Protease Inhibitor (PZ/ZPI) Anticoagulant System and Thrombosis. British Journal of Haematology. 2007. 137(2): 99-108.
[2] "Chapter 1: Blood Coagulation as a Part of the Haemostatic System." Blood Coagulation. Ed. R. F. A. Zwaal and H. C. Hemker. Vol. 13. New York: Elsevier Science, 1986. Elsevier Science Publishing Company (Biomedical Division).
[3] Huang, Xin et al. Basis for the Specificity and Activation of the Serpin Protein Z-dependent Proteinase Inhibitor (ZPI) as an Inhibitor of Membrane-associated Factor Xa. The Journal of Biological Chemistry. June 25, 2010. 285(26): 20399–20409.
[4] Wei, Zhenquan et al. Crystal Structure of Protein Z-Dependent Inhibitor Complex Shows How Protein Z Functions as a Cofactor in the Membrane Inhibition of Factor X. Blood. June 15, 2009. 114: 3662-3667.
[5] Lee, C. J. et al. A proposed structural model of human protein Z. Journal of Thrombosis and Haemostasis. July 2007. 5: 1558–1561.
[6] Sofi, Francesco et al. Protein Z: “Light and Shade” of a New Thrombotic Factor. Clinical Laboratories. 2004. 50. Department of Medical and Surgical Critical Care, Thrombosis Centre, University of Florence, Italy.
[7] RCSB Protein Data Bank: http://www.rcsb.org/pdb/explore/biologyAndChemistry.do?structureId=3F1S