Sandbox Reserved 598
From Proteopedia
| This Sandbox is Reserved from Feb 1, 2013, through May 10, 2013 for use in the course "Biochemistry" taught by Irma Santoro at the Reinhardt University. This reservation includes Sandbox Reserved 591 through Sandbox Reserved 599. |
To get started:
More help: Help:Editing |
Contents |
Background
Janus Kinase 2 is a non-receptor janus kinase, a protein which is part of the tyrosine kinases. These group of kinases are the primary intracellular mediators of cytokine signaling and are involved in the control of cellular growth. As a non-receptor kinase, Jak 2 has a cytoplasmic enzyme which catalyzes the transfer of a phosphate group through phosphorylation to the tyrosine residue in the protein. Such an enzyme plays a crucial role in regulating various cellular functions by switching on or off additional enzymes within the cell. [1] Such phosphorylation is a reversible process, and used in many different pathways as a method to control cellular activity. However kinases like Jak2, have enzymes which add phosphate groups to hydroxyl side chains as can be seen in the diagram. [2]
Jak2 was given its name "Janus" after the two-faced Roman God "Janus" who was known as the custodian of the universe and the God of new beginnings. [3] The abbreviation 'Jak' is commonly referred to as 'just another kinase' as, when it was first discovered, the kinase's role was not yet fully understood. [4]
Analyzing and Discovering Jak2 Structure
In determining the three-dimensional structure of the Jak2 protein, three primary methods of analysis were used; protein expression and purification, crystallization, and x-ray data collection. [5] In the protein expression and purification the Jak2 residue was cloned using pFastBac which uses “two promoters in a single vector for expression of two proteins simultaneously in insect cells”. [6] The bacmid DNA with the kinase insert was then isolated and put into cells of insect army worms. The cells were then grown, lysed and centrifuged after which the protein was then incubated, separated with gel filtration and fractions were taken for crystallization trials. In these crystallization trials, the protein residue was used to grow crystals via hanging drop vapor-diffusion. The purified protein complex was then mixed with solutions which subsequently formed crystals after one to three days. The crystalized protein was then flash frozen and the structure determined via molecular replacement and the AmoRe program. [7]
|
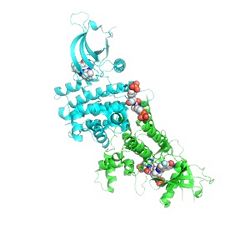
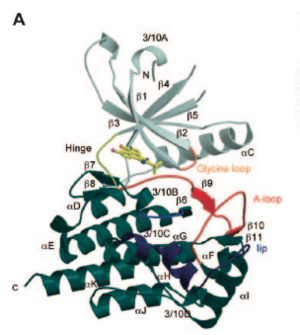
The structure of Jak 2 can be broken down into seven separate components, as seen in the pictured diagram to the left. The top, gray, portion of the protein was found to be the N-terminal lobe (residues 840-931). This loop is comprised of a 5-stranded anti-parallel beta-sheet (Beta1 - 5) and one alpha-helix (alpha C). The large dark green portion seen on the bottom is the COOH-terminal lobe. This carboxylic acid lobe is comprised of 8 alpha-helices (alpha D-alpha K), and 3 3/10 helices (3/10B, 3/10C, 3/10D), and 3 pairs of anti-parallel Beta-strands (Beta7-8, 6-9, and 10-11). The orange portion in the middle-right of the protein is the glycine loop which makes contacts with the activation loop and catalytic loop. This glycine loop, while small, is of great importance, as it is known to be essential in substrate and nucleotide binding. In yellow, to the top-left, there is a hinge region present which aids in molecule interactions. The blue section in the low middle is the catalytic loop, the red loop to the right is the activation loop and finally the dark blue section towards the bottom-right is the JAK2 lip which contains one 3/10C helix and one alpha-helix connected by a short linker. [8]
Function
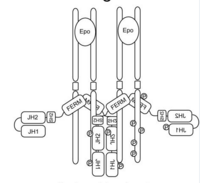
There are four members of the janus kinase family, Jak1, Jak2, Jak3, and Jak4. Each kinase has unique functions based on their respective abilities to bind to different cytokine receptors. Out of those four janus kinases, Jak2 is responsible for erythropoietin and thrombopoietin signaling which then causes the proliferation, activation and transcription of blood cells. [9] The Jak2 Signal Transducers and Activators of Transcription (STAT) pathway is what influences the kinase ability. The janus kinase-STAT pathway is the central path taken for such cell signal transcription though the erythropoietin receptor. [10] Under normal conditions, various levels of regulation maintain Jak2 in its inactive form until receptor activation occurs and involve interactions of the pseudokinase domain (JH2) and FERM domain (JH4 to -7) with the kinase domain (JH1). [11]
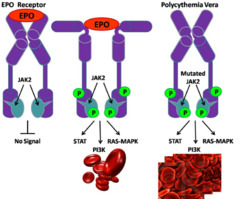
In the unbound and inactive state of Jak2, the FERM (JH4-7), JH1 (kinase domain) and JH2 (pseduokinase domain) domains of are tightly folded together preventing the catalytic domain from being active. (Figure 1) However, the first activation step of Jak2 is the displacement of the FERM domain by its interaction with the receptor which begins the unfolding of domains within the protein to allow access to the various binding domains. (Figure 2). In the presence of a ligand, receptor aggregation occurs. As a result of this aggregation, the tyrosine within the Jak2 active loop is phosphorylated and then induces changes in both JH2 and JH1 domains. Once phosphorylation occurs within the activation loop, the kinase is fully activated. (Figure 3) {Figures 1-3 from (Funakoshi-Tago, 2008)} Under normal conditions, Jak2 is not associated with a receptor and is locked into an inactive state. Receptor binding through the FERM domain relieves steric constraints, and allows Jak2 to be activated once it is engaged with its erythropoietin receptor. Activation of Jak2 by erythropoietin receptor engagement leads to the tyrosine phosphorylation of the receptor and Jak2. [12]
Mutations in Jak2 can result in the erythropoietin receptor being activated all the time. Due to lack of auto-inhibition of the JAK2 enzyme because of this activating mutation the receptor is effectively switched to the ‘on’ position indefinitely, constantly over-produce cells. This type of mutation has been found to occur in a nucleotide substitution of valine to phenylalanine and is therefore termed, V617F. The active conformation of Jak2 is likely to be mimicked by the Jak2-V617F mutant and this thereby results in either oncogenesis, polycythemia or other hematopoietic disorders. [13] Other mutant conformations, such as the Y613E mutant, failed to undergo complete conformational changes leading to its activation. [14] A diagramed example of how normal Jak2 receptors work, as well as how this mutation works mechanically can be seen on figure 4.
Figure 5 shows the amino acid sequence alignment of JAK2 along with the other members of the JAK family TYK2, JAK3, and JAK1 and the kinase domain of FAK and LCK around the Lip region. Cylinders show alpha-helices, the residues conserved among the JAK kinases sequence are highlighted in red. The dark gray boxes indicate which residues are conserved to at least 75% within the janus kinase family and finally the light gray boxes indicate conservatively substituted residues. [15]Conditions Associated with Jak2 Mutations
As Janus Kinase 2 has a significant role in hematopoiesis, the formation and development of blood cells, mutations in the protein most commonly result in constitutive kinase activation which lead to oncogenesis. Some of the cancers associated with such mutations have been found to be myeloid leukemia, lymphoid leukemia, polycythemia vera, along with other myeloproliferative neoplasms. [16] While there are many different genetic mutations which are resultant of leukemias, 85% of patients diagnosed with polycythemia vera are found to have the mutation in their Jak2 protein. [17]
Current and Future Therapies
There are currently many therapies for differing forms of leukemia, some of which include cytoreductive medications such as hydroxyurea or agrylin, to suppress the bone marrow’s ability to make blood cells, cell destructive medications like cytoxin which act as oral chemotheraputic agents, interferon treatments to stimulate the patient's immune response to fight and kill overproduction or white and red blood cells. Finally traditional chemotherapy is commonly used, as well, for both leukemias as well as progressive polycythemia vera. [18] [19] [20] [21] While there are a few Jak2 inhibitors already in use which use competitive inhibition for ATP binding pockets, they are not extremely effective due to non-specificity. Due to this issue with specificity, the current therapies for Jak2 mutations are being more focused on allosteric inhibition designs. This research is believed to be hopeful due to the successes it has had with other, different, kinase inhibition. Possible sites which scientists are targeting for such inhibition include, the type II Inhibitor pocket, substrate binding sites, kinase pseudo kinase domain interface, SH2JK2 Linker Region, and the FERM Domain. Currently many of these are in both pre and post clinical trials. [22] A brief discussion on diseases associated with Jak2, as well as the function of the protein can be found on an already established Proteopedia page found at 2b7a.
References
- ↑ Hanks, SK., Quinn, AM., Hunter, T. (1988). The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241 (4861): 42–52. doi:10.1126/science.3291115. PMID 3291115.
- ↑ Hudel, H. [Internet]. Irvine [CA]. Center for Biomembrane Systems at UC Irvine; c2013. [Updated 2013 Jan 3; cited 2013 March 23]. Available from: http://bass.bio.uci.edu
- ↑ Janus incarnate [Internet]. Kirwan Studios; c2011. [Updated 2011 Feb 3; cited 2013 March 23' Retrieved from http://rense.com/general92/janus.htm http://rense.com/general92/janus.htm
- ↑ Weinberg, I. (April 2010). Janus Kinase (Jak2)”. Vascular Medicind; Angiolgist http://www.angiologist.com/general-medicine/janus-kinase-2-jak2/
- ↑ Lucet, I., Fantino, E., & Styles, M. (2005). The structural basis of janus kinase 2 inhibition by a potent and specific pan-janus kinase inhibitor. Blood, 107, 176-183. doi: 10.1182/blood-2005-06-2413 http://bloodjournal.hematologylibrary.org/content/107/1/176.full.pdf
- ↑ pFastbac Duo [Internet]. Life Technologies Corporation; c2013. [Updated 2013; cited 2013 March 23]. Available from: http://www.invitrogen.com/1/1/14896-pfastbac-dual.html
- ↑ ) Lucet, I., Fantino, E., & Styles, M. (2005). The structural basis of janus kinase 2 inhibition by a potent and specific pan-janus kinase inhibitor. Blood, 107, 176-183. doi: 10.1182/blood-2005-06-2413 http://bloodjournal.hematologylibrary.org/content/107/1/176.full.pdf
- ↑ ) Lucet, I., Fantino, E., & Styles, M. (2005). The structural basis of janus kinase 2 inhibition by a potent and specific pan-janus kinase inhibitor. Blood, 107, 176-183. doi: 10.1182/blood-2005-06-2413 http://bloodjournal.hematologylibrary.org/content/107/1/176.full.pdf
- ↑ Weinberg, I. (April 2010). Janus Kinase (Jak2)”. Vascular Medicind; Angiolgist http://www.angiologist.com/general-medicine/janus-kinase-2-jak2/
- ↑ O'Shea, J., Gadina, M., & Chen, X. (2005). Structure of a janus kinase: molecular insights and prospects for optimizing a new class of immunosuppressants. The Journal of the American Society of Hematology, 106(3), 765-766. doi: 10.1182/blood-2005-05-1947 http://bloodjournal.hematologylibrary.org/content/106/3/765.full
- ↑ Funakoshi-Tago, M., Pelletier, S., & Moritake, H. (2008). Jak2 ferm domain interaction with the erythropoietin receptor regulates jak2 kinase activity. Molecular and Cellular Biology, 28(5), 1792-1801. doi: 10.1128/MCB.01447-07 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2258779/
- ↑ Funakoshi-Tago, M., Pelletier, S., & Moritake, H. (2008). Jak2 ferm domain interaction with the erythropoietin receptor regulates jak2 kinase activity. Molecular and Cellular Biology, 28(5), 1792-1801. doi: 10.1128/MCB.01447-07 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2258779/
- ↑ Gnanasambandan, K., & Sayeski, P. (2011). A structure-function perspective of jak2 mutations and implications for alternate drug design strategies: the road not taken. Department of Physiology and Functional Genomics, University of Florida College of Medicine, 18(30), 59-73. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21864276 http://www.ncbi.nlm.nih.gov/pubmed/21864276
- ↑ Funakoshi-Tago, M., Pelletier, S., & Moritake, H. (2008). Jak2 ferm domain interaction with the erythropoietin receptor regulates jak2 kinase activity. Molecular and Cellular Biology, 28(5), 1792-1801. doi: 10.1128/MCB.01447-07 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2258779/ (7) Kirwan, J. (02, 2011 03). Janus incarnate. Retrieved from http://rense.com/general92/janus.htm
- ↑ ) Lucet, I., Fantino, E., & Styles, M. (2005). The structural basis of janus kinase 2 inhibition by a potent and specific pan-janus kinase inhibitor. Blood, 107, 176-183. doi: 10.1182/blood-2005-06-2413 http://bloodjournal.hematologylibrary.org/content/107/1/176.full.pdf
- ↑ Funakoshi-Tago, M., Pelletier, S., & Moritake, H. (2008). Jak2 ferm domain interaction with the erythropoietin receptor regulates jak2 kinase activity. Molecular and Cellular Biology, 28(5), 1792-1801. doi: 10.1128/MCB.01447-07 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2258779/
- ↑ Polycythemia Vera [Internet]. Rochester [Mn]. The Mayo Clinic; c2011. [Updated 2011 Apr 7; cited 2013 March 23]. Available from: http://www.mayoclinic.com/health/polycythemia-vera/DS00919
- ↑ Acute Myeloid Leukemia [Internet]. Bethesda [MD]. The National Cancer Institute; c2013. [Updated 2013 March 06; cited 2013 March 23]. Available from: http://m.cancer.gov/topics/treatment/bycancer/adultAML/Patient
- ↑ Chronic Lymphocytic Leukemia [Internet]. Rochester [Mn]. The Mayo Clinic; c2011. [Updated 2011 Apr 7; cited 2013 March 23]. Available from: http://www.mayoclinic.com/health/chronic-lymphocytic-leukemia/DS00565
- ↑ Polycythemia Vera [Internet]. Rochester [Mn]. The Mayo Clinic; c2011. [Updated 2011 Apr 7; cited 2013 March 23]. Available from: http://www.mayoclinic.com/health/polycythemia-vera/DS00919
- ↑ Polycythemia Treatment and Management [Internet]. Medscape; 2012. [Updated 2012 Jan 10; cited 2013 March 23]. Available from: http://emedicine.medscape.com/article/205114-treatment
- ↑ Gnanasambandan, K., & Sayeski, P. (2011). A structure-function perspective of jak2 mutations and implications for alternate drug design strategies: the road not taken. Department of Physiology and Functional Genomics, University of Florida College of Medicine, 18(30), 59-73. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21864276 http://www.ncbi.nlm.nih.gov/pubmed/21864276