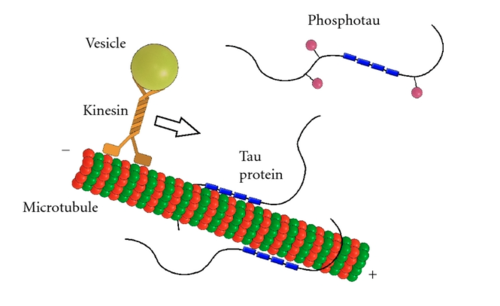
Tau protein
From Proteopedia
References
- ↑ 1.0 1.1 1.2 1.3 Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006247. doi:, 10.1101/cshperspect.a006247. PMID:22762014 doi:http://dx.doi.org/10.1101/cshperspect.a006247
- ↑ 2.0 2.1 Schweers O, Schonbrunn-Hanebeck E, Marx A, Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994 Sep 30;269(39):24290-7. PMID:7929085
- ↑ 3.0 3.1 3.2 Lei P, Ayton S, Finkelstein DI, Adlard PA, Masters CL, Bush AI. Tau protein: relevance to Parkinson's disease. Int J Biochem Cell Biol. 2010 Nov;42(11):1775-8. doi:, 10.1016/j.biocel.2010.07.016. Epub 2010 Aug 1. PMID:20678581 doi:http://dx.doi.org/10.1016/j.biocel.2010.07.016
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Buee, L, Bussiere, T, Buee-Scherrer, V, Delacourte, A, Hof, PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 33:95-130 (2000). DOI: 10.1016/S0165-0173(00)00019-9
- ↑ 5.0 5.1 5.2 5.3 5.4 Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012:731526. doi: 10.1155/2012/731526. Epub 2012 May, 29. PMID:22690349 doi:http://dx.doi.org/10.1155/2012/731526
- ↑ Qurashi I, Collins J, Chaudhry I, Husain N. Promising use of minocycline augmentation with clozapine in treatment-resistant schizophrenia. J Psychopharmacol. 2014 Mar 19;28(7):707-708. PMID:24646811 doi:http://dx.doi.org/10.1177/0269881114527358
- ↑ 7.0 7.1 7.2 Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991 Nov;115(3):717-30. PMID:1918161
- ↑ Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004 Aug 13;279(33):34873-81. Epub 2004 Jun 9. PMID:15190058 doi:http://dx.doi.org/10.1074/jbc.M405131200
- ↑ Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004 Jul;10 Suppl:S10-7. PMID:15272267 doi:http://dx.doi.org/10.1038/nm1066
- ↑ Biernat J, Mandelkow EM, Schroter C, Lichtenberg-Kraag B, Steiner B, Berling B, Meyer H, Mercken M, Vandermeeren A, Goedert M, et al.. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992 Apr;11(4):1593-7. PMID:1563356
- ↑ Cohen TJ, Friedmann D, Hwang AW, Marmorstein R, Lee VM. The microtubule-associated tau protein has intrinsic acetyltransferase activity. Nat Struct Mol Biol. 2013 Jun;20(6):756-62. doi: 10.1038/nsmb.2555. Epub 2013 Apr, 28. PMID:23624859 doi:http://dx.doi.org/10.1038/nsmb.2555
- ↑ Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):7501-6. doi:, 10.1073/pnas.1504081112. Epub 2015 Jun 1. PMID:26034266 doi:http://dx.doi.org/10.1073/pnas.1504081112
- ↑ 13.0 13.1 Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004 Jul;10 Suppl:S10-7. PMID:15272267 doi:http://dx.doi.org/10.1038/nm1066
- ↑ Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003 Apr;62(4):389-97. PMID:12722831
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Madelyn Kasprzak, Jaime Prilusky