This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Stephanie Miller/Sandbox32
From Proteopedia
This page is the sandbox. Use it for making test edits. You may delete everything on this page (but please leave this introductory paragraph).
Contents |
Cyan Fluorescent Protein (CFP)
Background
The protein responsible for the bioluminescence also known as Green Fluorescent Protein was discovered by Osamu Shimomurain Aequorea Victoria in 1962. GFP was found to fluoresce green under blue light excitation, requiring no substrates or coenzymes. Its excitation spectrum has a dominant maximum at ~ 400 nm and a smaller maximum at ~ 470 nm. The emission spectrum has a sharp maximum at ~ 505 nm and a shoulder at ~ 540 nm. The structure of this protein consists of 238 amino acids, an 11-stranded β-barrel threaded by an α-helix (where the chromophore is located), which then runs up along the axis of the cylinder.
Since its discovery, GFP has revolutionized the field of science and has allowed scientists to tag cells. As a protein tag, GFP is capable of tracking and quantifying individual or multiple protein species. GFP serves as probes to monitor protein-protein interactions in a cell. It is a biosensors to describe biological events and signals, etc. All of these functions help us view tagged cells in vivo. By understanding the interaction between proteins in vivo, we can identify the complex pathway of a particular protein. Additionally, many mutants of GFP have been collected and studied; the importance of these GFP derivatives is that scientists are able to trace various types of cells at the same time in vitro.
|
Structure
The chromophore, which contains the Ser65-Tyr66-Gly67, is stabilized by the that surrounds the structure. GFP contains only one amino acid (Tyr66) in the chromophore (Tyrosine(green) of the Chromophore). Simple modifications to this sequence can allow GFP to undergo several different color changes. When Tyr66, in the same position of the chromophore, changes into Tryptophan the color of GFP changes to Cyan Fluorescent Protein(CFP). This change allows for the protein to fluoresce blue. The Tyr 66 in the GFP molecule plays a primary role in stabilizing the green fluorescent. This is due to the aromatic rings that the amino acid contains. Likewise, Tryptophan contains aromatic rings. In the PDB model 1oxe of CPF, we highlighted the specific amino acid that is pertinent in color change from green to blue. The amino acid, , is colored dark blue in the rasmol model. Along with tryptophan, we used a purple color to display the nitrogen in the tryptophan indole ring. A minute change in amino acid Tyr66 allowed for the fluorescent protein to go from green to blue.
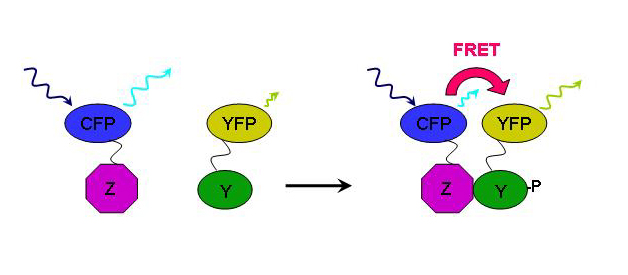
Applications
Picture by 
GFP has an important role in biology in which it is used to color tag important biological proteins. With CFP scientist can can also color tag a protein they wish to investigate, however a property of CFP allows you to see protein interactions with the help of YFP. This application of CFP is call FRET (Fluorescent Resonance Energy Transfer). When scientist excites CFP with a UV light at ~436nm, it emits a wavelength at ~480nm which overlaps the excitation spectrum of YFP. When a protein with CFP is nearby a protein tagged with YFP, a green glow will be observed which is the combination of blue (CFP) and yellow (YFP).