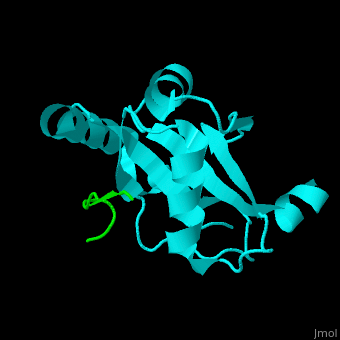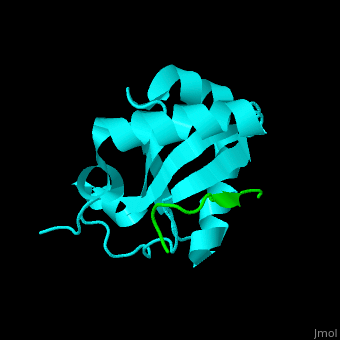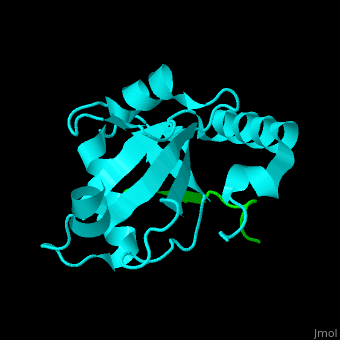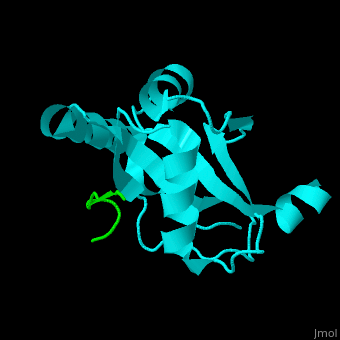Microtubule-associated protein
From Proteopedia
(Difference between revisions)
| (16 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='Human microtubule-associated protein light chain 3B (cyan) complex with sequestosome peptide (green) (PDB code [[2zjd]])' scene='70/708075/Cv/1'> |
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
| + | '''Microtubule-associated protein light chain 3''' (LC3) is recruited to autophagosomal membranes during autophagy<ref>PMID:7480164</ref>. The human LC3B is cleaved after synthesis to expose a C-terminal glycine which binds via a phospholipid anchor to autophagosomal vesicle membranes during autophagy. Thus detection of LC3 is a reliable method for monitoring autophagy. The microtubule-associated proteins are classified as 2 types. Type 1 includes MAP1 and type 2 includes MAP2, MAP4 and [[Tau protein]]. | ||
| + | |||
| + | *'''MAP1''' are classical microtubule-associated proteins which bind along microtubule lattice<ref>PMID:16938900</ref>.<br /> | ||
| + | *'''Tau protein''' is the principal component of the tangles found in Alzheimer's disease. It is hyperphosphorylated on serines and threonines<ref>PMID:19542604</ref>. | ||
| + | *'''Microtubule-associated protein RP/EB family member 1''' (MAPRE1) is involved in the regulation of microtubule structure and chromosome stability. It associates with dynactin and the spindle during mitosis<ref>PMID:22919</ref>. | ||
| + | *'''Microtubule-associated protein RP/EB family member 3''' (MAPRE3) localizes to the cytoplasmic microtubule network and binds a homolog of the adenomatous polyposis coli tumor suppressor gene<ref>PMID:22924</ref>. | ||
== Disease == | == Disease == | ||
| - | + | '''Microtubule-associated protein Tau''' is associated with Alzheimer Disease. Tau forms abnormal aggregates in patients' tissues. Mutations in tau protein are associated with neurodegenerative diseases like frontotemporal dementia<ref>PMID:15056452</ref>. | |
| - | + | ||
== Structural highlights == | == Structural highlights == | ||
| + | <scene name='70/708075/Cv/5'>LC3 recognizes a sequestosome peptide which is a preferred target for autophagy and binds at the surface of LC3</scene><ref>PMID:18524774</ref>. Water molecules are shown as red spheres. | ||
| + | |||
| + | == 3D Structures of microtubule-associated protein == | ||
| + | [[Microtubule-associated protein 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
| - | == 3D Structures of microtubule-associated protein light chain 3 == | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | |||
| - | *MAP1 light chain 3A | ||
| - | |||
| - | **[[1ugm]] – hLC3 – human<br /> | ||
| - | **[[3eci]], [[3wal]] – hLC3A<br /> | ||
| - | |||
| - | *MAP1 light chain 3B | ||
| - | |||
| - | **[[3vtu]] – hLC3B<br /> | ||
| - | **[[1v49]] – hLC3B – NMR<br /> | ||
| - | **[[3vtv]], [[3vtw]] – hLC3B/optineurin (mutant)<br /> | ||
| - | **[[2lue]] – hLC3B + optineurin peptide<br /> | ||
| - | **[[2zjd]] – hLC3B + sequestosome p62 peptide<br /> | ||
| - | **[[2z0d]], [[2z0e]], [[2zzp]] – rLC3B + hCysteine protease ATG4B – rat<br /> | ||
| - | **[[2k6q]] – rLC3B + sequestosome p62 peptide - NMR<br /> | ||
| - | |||
| - | *MAP1 light chain 3C | ||
| - | |||
| - | **[[3wam]] – hLC3C<br /> | ||
| - | **[[3vvw]] – hLC3C + NDP52<br /> | ||
| - | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev. 1995 Oct;75(4):835-64. PMID:7480164
- ↑ Halpain S, Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol. 2006;7(6):224. PMID:16938900
- ↑ Lebouvier T, Scales TM, Williamson R, Noble W, Duyckaerts C, Hanger DP, Reynolds CH, Anderton BH, Derkinderen P. The microtubule-associated protein tau is also phosphorylated on tyrosine. J Alzheimers Dis. 2009;18(1):1-9. doi: 10.3233/JAD-2009-1116. PMID:19542604 doi:http://dx.doi.org/10.3233/JAD-2009-1116
- ↑ Buligescu L, Lenkei R, Ciontea M, Dan EM. [Significance of anti-albumin antibodies in chronic liver disease]. Rev Med Interna Neurol Psihiatr Neurochir Dermatovenerol Med Interna. 1977, Jul-Aug;29(4):363-70. PMID:22919
- ↑ Turtola LO. Enamel microhardness and fluoride uptake underneath fermenting and non-fermenting artificial plaque. Scand J Dent Res. 1977 Sep;85(6):373-9. PMID:22924
- ↑ Schraen-Maschke S, Dhaenens CM, Delacourte A, Sablonniere B. Microtubule-associated protein tau gene: a risk factor in human neurodegenerative diseases. Neurobiol Dis. 2004 Apr;15(3):449-60. PMID:15056452 doi:http://dx.doi.org/10.1016/j.nbd.2003.12.009
- ↑ Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, Kominami E, Yamane T, Tanaka K, Komatsu M. Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem. 2008 Aug 15;283(33):22847-57. Epub 2008 Jun 4. PMID:18524774 doi:http://dx.doi.org/M802182200
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky