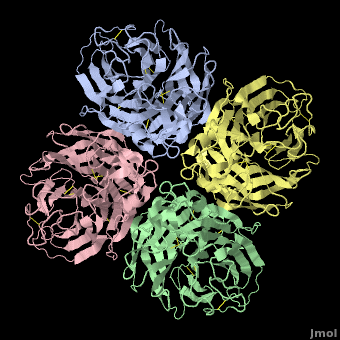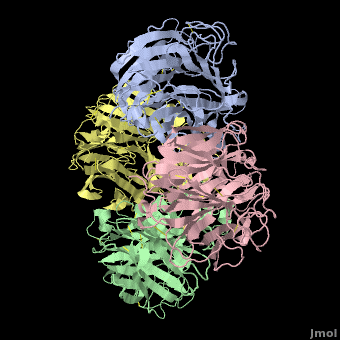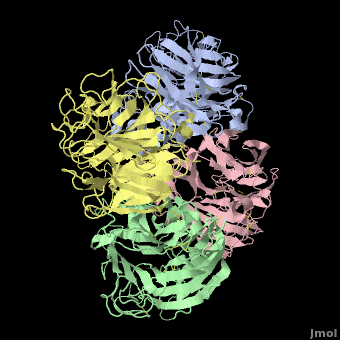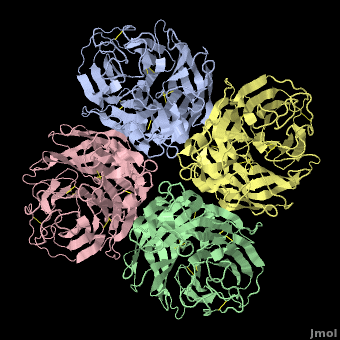Avian Influenza Neuraminidase, Tamiflu and Relenza
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='2hu4_1.pdb' size='450' side='right' scene='Avian_Influenza_Neuraminidase,_Tamiflu_and_Relenza/2hu4_tetramer/1' caption='right' caption='Influenza Neuraminidase N1.'> | ||
| + | |||
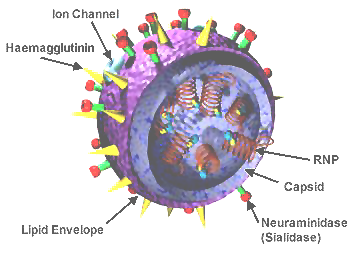
[[Influenza]] is a contagious disease caused by a virus. Influenza A<ref name="flu_a">See [http://en.wikipedia.org/wiki/Influenza#Types_of_influenza_virus Influenza A Types of Influenza Virus] (in Wikipedia).</ref> (one of several genera and species of influenza) is the most virulent form infecting humans. Largely by facilitating secondary bacterial pneumonias, influenza kills 500,000 people worldwide annually (including about 36,000 in the USA), mostly during seasonal [http://en.wikipedia.org/wiki/Epidemic epidemics] each year. Most people killed in the annual influenza epidemics are people whose immune defenses are weak, including the very young and the old. Influenza also kills large numbers of animals and birds, both domestic and wild<ref name="animals">[http://www.fao.org/avianflu/en/clinical.html Epidemiology of Avian Influenza] at the [http://fao.org Food and Agriculture Organization of the United Nations].</ref>. The influenza virus includes only eight proteins. Sequences of these proteins as obtained from numerous strains are available in the [http://www.ncbi.nlm.nih.gov/genomes/FLU/Database/select.cgi?go=1 NCBI Influenza Virus Resource]. For more about the structure and biology, including references for the points made here, please see [http://en.wikipedia.org/wiki/Influenza Influenza at Wikipedia]. | [[Influenza]] is a contagious disease caused by a virus. Influenza A<ref name="flu_a">See [http://en.wikipedia.org/wiki/Influenza#Types_of_influenza_virus Influenza A Types of Influenza Virus] (in Wikipedia).</ref> (one of several genera and species of influenza) is the most virulent form infecting humans. Largely by facilitating secondary bacterial pneumonias, influenza kills 500,000 people worldwide annually (including about 36,000 in the USA), mostly during seasonal [http://en.wikipedia.org/wiki/Epidemic epidemics] each year. Most people killed in the annual influenza epidemics are people whose immune defenses are weak, including the very young and the old. Influenza also kills large numbers of animals and birds, both domestic and wild<ref name="animals">[http://www.fao.org/avianflu/en/clinical.html Epidemiology of Avian Influenza] at the [http://fao.org Food and Agriculture Organization of the United Nations].</ref>. The influenza virus includes only eight proteins. Sequences of these proteins as obtained from numerous strains are available in the [http://www.ncbi.nlm.nih.gov/genomes/FLU/Database/select.cgi?go=1 NCBI Influenza Virus Resource]. For more about the structure and biology, including references for the points made here, please see [http://en.wikipedia.org/wiki/Influenza Influenza at Wikipedia]. | ||
| Line 9: | Line 11: | ||
For more about neuraminidase, including references for the points made in this paragraph, please see [http://en.wikipedia.org/wiki/Influenza Influenza at Wikipedia]. | For more about neuraminidase, including references for the points made in this paragraph, please see [http://en.wikipedia.org/wiki/Influenza Influenza at Wikipedia]. | ||
{{Clear}} | {{Clear}} | ||
| - | <applet load='2hu4_1.pdb' size='500' frame='true' align='right' caption='Influenza Neuraminidase N1 (2hu4).' scene='Avian_Influenza_Neuraminidase,_Tamiflu_and_Relenza/2hu4_tetramer/1' /> | ||
===Neuraminidase Structure and Conserved Amino Acids=== | ===Neuraminidase Structure and Conserved Amino Acids=== | ||
| Line 58: | Line 59: | ||
==Prophylaxis and Treatment of Influenza== | ==Prophylaxis and Treatment of Influenza== | ||
| - | '''Vaccines''' are effective at preventing influenza, but only if they target the relevant viral subtypes. New vaccines against the annual epidemics of influenza A and B are prepared each year, separately in the northern and southern hemispheres. These are designed to target the subtypes predicted to be prevalent in any given flu season, but sometimes those predictions are wrong, leading to that year's vaccine being ineffective. A vaccine for a pandemic strain of H5N1 could not be prepared until after the pandemic began, because only then would the relevant subtype be known<ref>[http://en.wikipedia.org/wiki/Influenza#Vaccination_and_infection_control Vaccination for Influenza] at Wikipedia</ref>. | + | '''Vaccines''' are effective at preventing influenza, but only if they target the relevant viral subtypes. New vaccines against the annual epidemics of influenza A and B are prepared each year, separately in the northern and southern hemispheres. These are designed to target the subtypes predicted to be prevalent in any given flu season, but sometimes those predictions are wrong, leading to that year's vaccine being ineffective. A vaccine for a pandemic strain of H5N1 could not be prepared until after the pandemic began, because only then would the relevant subtype be known<ref>[http://en.wikipedia.org/wiki/Influenza#Vaccination_and_infection_control Vaccination for Influenza] at Wikipedia</ref>. Various formulations of vaccines are available according to the ages of the recipients<ref>[http://onlinenursepractitionerprograms.com/2012/part-ii-what-you-need-to-know-about-2012-2013-flu-vaccine/ What You Need to Know About 2012-2013 Flu Vaccine].</ref> |
[[Pharmaceutical_Drugs#Treatments|Drugs against influenza]], stockpiled in advance of a panedmic, appear to be the best preparation, given the limitations of vaccines. Tens of billions of dollars have been spent on pandemic preparedness in the USA alone, and a large portion of these expenditures is for [http://en.wikipedia.org/wiki/Oseltamivir stockpiling of anti-influenza drugs]. Similar expenditures have been made in many developed countries. The World Health Organization is poised to distribute anti-influenza drugs at the first signs of an epidemic of H5N1. | [[Pharmaceutical_Drugs#Treatments|Drugs against influenza]], stockpiled in advance of a panedmic, appear to be the best preparation, given the limitations of vaccines. Tens of billions of dollars have been spent on pandemic preparedness in the USA alone, and a large portion of these expenditures is for [http://en.wikipedia.org/wiki/Oseltamivir stockpiling of anti-influenza drugs]. Similar expenditures have been made in many developed countries. The World Health Organization is poised to distribute anti-influenza drugs at the first signs of an epidemic of H5N1. | ||
| Line 75: | Line 76: | ||
====Resistance to Tamiflu and Relenza==== | ====Resistance to Tamiflu and Relenza==== | ||
| - | < | + | |
| - | Because Tamiflu and Relenza closely resemble the natural sialic acid substrate of neuraminidase, it was hoped that mutations conferring resistance to these drugs would greatly lower the virulence of influenza carrying such mutations. This hope has proven false in the case of Tamiflu<ref name="collins2008">PMID:18480754</ref>. | + | <scene name='User:Eric_Martz/Molecular_Playground/Authoring/3ckz_relenza_tyr274/7'>Relenza binding to N1 mutant H274Y</scene> ([[3ckz]]). |
| + | |||
| + | Because Tamiflu and Relenza closely resemble the natural sialic acid substrate of neuraminidase, it was hoped that mutations conferring resistance to these drugs would greatly lower the virulence of influenza carrying such mutations. This hope has proven false in the case of Tamiflu<ref name="collins2008">PMID:18480754</ref>. Secondary mutations restore viral fitness<ref>PMID: 20522766</ref><ref>PMID: 20522774</ref>. By early 2009, 98% of influenza A/H1N1 strains circulating in North America had become resistant to Tamiflu<ref>PMID: 19299601</ref>. | ||
Two common mutations that confer resistance to Tamiflu did not confer resistance to Relenza<ref name="collins2008" />. At right is Relenza binding to the H274Y mutant of N1. (Wild type amino acids within 3 Å of Relenza are shown as thin sticks. <scene name='User:Eric_Martz/Molecular_Playground/Authoring/3ckz_relenza_tyr274/7'>Zoom out and back in</scene>.) This suggests that it would be prudent to stockpile Relenza in addition to Tamiflu, and that combination therapy might be the most effective weapon against a new pandemic, prior to development and deployment of a vaccine. | Two common mutations that confer resistance to Tamiflu did not confer resistance to Relenza<ref name="collins2008" />. At right is Relenza binding to the H274Y mutant of N1. (Wild type amino acids within 3 Å of Relenza are shown as thin sticks. <scene name='User:Eric_Martz/Molecular_Playground/Authoring/3ckz_relenza_tyr274/7'>Zoom out and back in</scene>.) This suggests that it would be prudent to stockpile Relenza in addition to Tamiflu, and that combination therapy might be the most effective weapon against a new pandemic, prior to development and deployment of a vaccine. | ||
| Line 85: | Line 88: | ||
====Tamiflu Binds to N1 by Induced Fit==== | ====Tamiflu Binds to N1 by Induced Fit==== | ||
| - | < | + | <scene name='Avian_Influenza_Neuraminidase,_Tamiflu_and_Relenza/Morph_2hty_to_2hu4/2'>Morph of N1 alone to N1 complexed with Tamiflu. </scene> The position where Tamiflu will bind is shown translucent except when bound in the empirically-determined model. N1 alone - ([[2hty]]), N1 complexed with Tamiflu - ([[2hu4]]). If the morph does not animate automatically, click this button: {{Template:Button Toggle Animation2}} |
| + | |||
Tamiflu was designed to fit N2/N9, so it is serendipitous that it works on N1. In fact, when the structure of N1 was determined<ref name='Russell2006' />, the <font color='#e07000'><b>loop comprising residues 147-152</b></font> was not in a suitable position to participate in binding Tamiflu. However, the complex of N1 with Tamiflu revealed that this loop is pulled into proper contact with the drug in an [[Induced fit|induced fit]] manner<ref name='Russell2006' />. A [[Morphs|morph]] from N1 alone ([[2hty]]) to N1 complexed with Tamiflu ([[2hu4]])<ref>Chain A from [[2hty]] was [[morphs|morphed]] to chain A of [[2hu4]] by linear interpolation, inserting 6 intermediate interpolated frames, using the freely available [http://www.umass.edu/microbio/rasmol/pdbtools.htm#martz morph2 program].</ref> shows the change in position of this loop (<scene name='Avian_Influenza_Neuraminidase,_Tamiflu_and_Relenza/Morph_2hty_to_2hu4/8'>replay initial morph</scene>). | Tamiflu was designed to fit N2/N9, so it is serendipitous that it works on N1. In fact, when the structure of N1 was determined<ref name='Russell2006' />, the <font color='#e07000'><b>loop comprising residues 147-152</b></font> was not in a suitable position to participate in binding Tamiflu. However, the complex of N1 with Tamiflu revealed that this loop is pulled into proper contact with the drug in an [[Induced fit|induced fit]] manner<ref name='Russell2006' />. A [[Morphs|morph]] from N1 alone ([[2hty]]) to N1 complexed with Tamiflu ([[2hu4]])<ref>Chain A from [[2hty]] was [[morphs|morphed]] to chain A of [[2hu4]] by linear interpolation, inserting 6 intermediate interpolated frames, using the freely available [http://www.umass.edu/microbio/rasmol/pdbtools.htm#martz morph2 program].</ref> shows the change in position of this loop (<scene name='Avian_Influenza_Neuraminidase,_Tamiflu_and_Relenza/Morph_2hty_to_2hu4/8'>replay initial morph</scene>). | ||
| - | The binding of Tamiflu to N1 pulls the sidechains of two conserved residues, <scene name='Avian_Influenza_Neuraminidase,_Tamiflu_and_Relenza/Morph_2hty_to_2hu4/7'>Asp151, Glu119</scene>, closer to the inhibitor. | + | The binding of Tamiflu to N1 pulls the sidechains of two conserved residues, <scene name='Avian_Influenza_Neuraminidase,_Tamiflu_and_Relenza/Morph_2hty_to_2hu4/7'>Asp151, Glu119</scene><!--(<font color="red">Sorry, this scene is temporarily broken.</font>)-->, closer to the inhibitor. |
===Cavity in N1: An Opportunity for Drug Design=== | ===Cavity in N1: An Opportunity for Drug Design=== | ||
| Line 109: | Line 113: | ||
*[http://www.pdb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb113_1.html Influenza Neuraminidase] in the [http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] series by [[User:David S. Goodsell|David S. Goodsell]]. This article includes visualization in Jmol of an alignment between wild-type and mutant neuraminidases with ligands sialic acid and oseltamivir (Tamiflu). [http://www.pdb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb113_jmol.html See Alignment]. | *[http://www.pdb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb113_1.html Influenza Neuraminidase] in the [http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] series by [[User:David S. Goodsell|David S. Goodsell]]. This article includes visualization in Jmol of an alignment between wild-type and mutant neuraminidases with ligands sialic acid and oseltamivir (Tamiflu). [http://www.pdb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb113_jmol.html See Alignment]. | ||
* For additional information, see: [[Influenza]] | * For additional information, see: [[Influenza]] | ||
| + | *[[Journal:Acta Cryst D:S2059798320011869|Lattice-translocation defects in some specific crystals of the catalytic head domain of influenza neuraminidase]] | ||
| + | |||
| + | ==3D structures of Neuraminidase== | ||
| + | [[Neuraminidase]] | ||
| + | </StructureSection> | ||
==Links== | ==Links== | ||
Current revision
| |||||||||||
Links
- cdc.gov/flu, the official influenza resource of the US Center for Disease Control.
- Pandemic Planning Toolkit (by Roche).
- Relenza offical website by GlaxoSmithKline.
- Tamiflu official website by Roche.
- The Next Pandemic? An authoritative overview of economic and political factors written in 2005 by Laurie Garrett.
Notes and Literature References
- ↑ See Influenza A Types of Influenza Virus (in Wikipedia).
- ↑ Epidemiology of Avian Influenza at the Food and Agriculture Organization of the United Nations.
- ↑ Image of influenza virus structure was obtained from Wikipedia.
- ↑ Influenza at Wikipedia.
- ↑ The tetramer is one of two biological units in the asymmetric unit of 2hu4.
- ↑ See Evolutionary Conservation. Coloring by ConSurf on chain A of 2hu4 based on 100 unique homologs using default conditions, done on September 23, 2008.
- ↑ Severe acute respiratory syndrome, SARS, was a near-pandemic in 2002-2003: see Severe Acute Respiratory Syndrome (in Wikipedia).
- ↑ 2009 Swine Flu Outbreak (in Wikipedia).
- ↑ Human Swine Influenza Investigation, April 23, 2009.
- ↑ From the CDC: Investigation and Interim Recommendations: Swine Influenza (H1N1).
- ↑ CDC document on Antiviral Drugs and Swine Influenza.
- ↑ Monto AS. Epidemiology of influenza. Vaccine. 2008 Sep 12;26 Suppl 4:D45-8. PMID:19230159
- ↑ 13.0 13.1 Some immunity to novel H1N1 flu found in seniors (May 21, 2009, University of Minnesota Center for Infectious Disease and Policy).
- ↑ 14.0 14.1 Morbidity and Mortality Weekly Report for May 22, 2009 from the US Centers for Disease Control and Prevention.
- ↑ Influenza Pandemic at Wikipedia.
- ↑ Vaccination for Influenza at Wikipedia
- ↑ What You Need to Know About 2012-2013 Flu Vaccine.
- ↑ Report on amantadine resistance, CDC Morbidity and Mortality Weekly Reports, January 2006.
- ↑ 19.0 19.1 19.2 19.3 Russell RJ, Haire LF, Stevens DJ, Collins PJ, Lin YP, Blackburn GM, Hay AJ, Gamblin SJ, Skehel JJ. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature. 2006 Sep 7;443(7107):45-9. Epub 2006 Aug 16. PMID:16915235 doi:http://dx.doi.org/10.1038/nature05114
- ↑ Varghese JN, Laver WG, Colman PM. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5-11;303(5912):35-40. PMID:6843658
- ↑ 21.0 21.1 Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Skehel JJ, Martin SR, Hay AJ, Gamblin SJ. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature. 2008 Jun 26;453(7199):1258-61. Epub 2008 May 14. PMID:18480754 doi:http://dx.doi.org/10.1038/nature06956
- ↑ Holmes EC. Virology. Helping the resistance. Science. 2010 Jun 4;328(5983):1243-4. PMID:20522766 doi:10.1126/science.1190994
- ↑ Bloom JD, Gong LI, Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science. 2010 Jun 4;328(5983):1272-5. PMID:20522774 doi:10.1126/science.1187816
- ↑ Layne SP, Monto AS, Taubenberger JK. Pandemic influenza: an inconvenient mutation. Science. 2009 Mar 20;323(5921):1560-1. PMID:19299601 doi:http://dx.doi.org/323/5921/1560
- ↑ Chain A from 2hty was morphed to chain A of 2hu4 by linear interpolation, inserting 6 intermediate interpolated frames, using the freely available morph2 program.
Proteopedia Page Contributors and Editors (what is this?)
Eric Martz, Eran Hodis, David Canner, Ilan Samish, Alexander Berchansky, Jaime Prilusky, David S. Goodsell, Michal Harel, Michael Strong