This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox reserved 659
From Proteopedia
(→Beta Lactoglobulin) |
|||
| (44 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== Beta Lactoglobulin == | == Beta Lactoglobulin == | ||
| - | [[Image:1b8e_bio_r_500.jpg]] | + | [[Image:1b8e_bio_r_500.jpg|thumb|left|260px|]] |
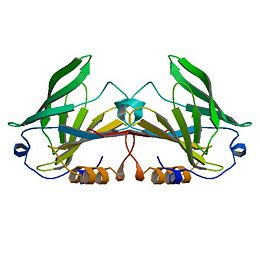
| - | β-Lactoglobulin (β-LG) is the | + | β-Lactoglobulin(β-LG) is the bulk constituent of whey protein in most ruminant species and is also present in the milks of most mammalian species, although not in rodent, human, and lagomorph milks. It has been studied extensively for some time ( Tilley, 1960; Lyster, 1972; Kinsella and Whitehead, 1987; Hambling et al., 1992; Sawyer, 2003). β-LG is a lipocalin as is seen through its amino-acid sequence and structure (Flower et al., 2000). Lipocalins are a varied family which bind small hydrophobic ligands, and often act as transporters in vivo. The purpose of β-LG, however, is not clear. Genetic variants of β-LG expressing genes have been reported, especially in cows and several nonruminant species (Kontopidis, 2004). |
| - | + | Whey is an often used food ingredient in the food industry and has many useful functional characteristics including foaming, gelling, thermal stability, emulsification, and protein fortification. It has also been suggested that it has many useful health benefits including exercise recovery, weight management, cardiovascular health, anti-cancer effects, anti-infection activity, wound repair, and infant nutrition. | |
| + | Human consumption of whey ingredients has steadily increased and has become more and more important to the dairy industry. | ||
| + | An issue with whey ingredients is the subtle off flavor often associated with whey proteins. During the | ||
| + | Cheddar cheese making process, norbixin leaches into the whey during the draining of the curd. Norbixin, a carotenoid extracted from the bixa orellana seed and added to cheese milk to give Cheddar its expected yellow flavor, would also contribute yellow color to the final product into which the whey ingredient is added. To prevent discoloration of the final product, whey is bleached by either hydrogen peroxide or benzoyl peroxide. In addition to a white color, this also causes lipid oxidation and associated off flavors. | ||
| + | |||
| + | [[Image:Picture2.jpg|thumb|right|260px|frame|Norbixin]] | ||
| + | |||
| + | [[Image:Picture6.jpg|thumb|right|260px|frame|Whey production process]] | ||
| + | |||
| + | [[Image:Picture4.png|thumb|left|260px|frame|Whey protein powder]] | ||
| + | |||
| + | [[Image:Picture3.png|thumb|center|610px|]] | ||
<StructureSection load='1B8E' size='350' side='right' caption='Structure of Beta Lactoglobulin (PDB entry [[1B8E]])' scene=''> | <StructureSection load='1B8E' size='350' side='right' caption='Structure of Beta Lactoglobulin (PDB entry [[1B8E]])' scene=''> | ||
| - | + | β-Lactoglobulin is a small protein with 162 amino acid residues (Mr ∼18,400). β-LG folds up into an 8-stranded, antiparallel β-barrel with a 3-turn α-helix on the outer surface and a ninth β-strand flanking the first strand. The ninth β-strand forms the dimer interface in cow and sheep proteins. The calyx, or β-barrel, is conical and serves as the ligand binding sight for small hydrophobic ligands. It is made of β-strands A-D forming one sheet, and strands E-H forming a second. Strand A turns at a right angle and the C-terminal end forms an antiparallel strand with H. A 3-turn helix between strands G and H is located on the outer surface of the β-barrel between strands G and H. The loops that connect the β-strands at the closed end of the calyx, BC, DE, and FG are generally quite short, whereas those at the open end are significantly longer and more flexible (Kontopidis, 2004). This becomes especially important when considering conformational changes at different pH values. Specifically, the EF loops can act as a “gate”, opening or closing access to and from the internal binding site. At acidic pH the “gate” closes while at neutral and basic pH values the “gate” is open, allowing for ligand binding at the hydrophobic binding site. The gate “hinge” is Glu89 (Kontopidis, 2004). | |
| + | |||
| + | The free Cys121 has a reactive thiol group, whose activity parallels the pH dependency of the Tanford transition and is involved with the denaturation and aggregation of the system (Havea et al., 2001).. It is situated on the outer surfaced of the β-barrel and underneath the α-helix, which offers it some protection from the solvent and pH effects. | ||
| + | |||
</StructureSection> | </StructureSection> | ||
| - | β-Lactoglobulin is a small protein, soluble in dilute salt solution as befits a globulin, with 162 amino acid residues (Mr ∼18,400) that fold up into an 8-stranded, antiparallel β-barrel with a 3-turn α-helix on the outer surface and a ninth β-strand flanking the first strand (see Figure 1). It is this strand that forms a significant part of the dimer interface in the bovine and ovine proteins but, while still present in porcine β-LG, is not involved in the formation of the dimer that forms at low pH. The genetic variants referred to above in the ruminant species result in relatively minor amino acid differences, but the processing properties appear to be significantly affected even by these small changes, such that consideration has been given to removing the less favorable variants by selective breeding (Hill et al., 1997; Harris, 1997). The so-called calyx, or β-barrel, is conical and is made of β-strands A-D forming one sheet, and strands E-H forming a second. Strand A bends through a right angle such that the C-terminal end forms an antiparallel strand with H; strands D and E also form a less significant interaction completely closing the calyx. It is this central cavity, the calyx, that provides the ligand-binding site. On the outer surface of the β-barrel, between strands G and H, is the 3-turn α-helix. The loops that connect the β-strands at the closed end of the calyx, BC, DE, and FG are generally quite short, whereas those at the open end are significantly longer and more flexible. | ||
| - | In particular, the EF loop acts as a gate over the binding site. At low pH, it is in the “closed” position, and binding is inhibited or impossible, whereas at high pH it is open, allowing ligands to penetrate into the hydrophobic binding site. The “latch” for this gate is Glu89, the residue implicated in the Tanford transition observed, although not identified, as having an abnormally high pKa (Tanford et al., 1959; Brownlow et al., 1997; Qin et al., 1998a). | ||
| - | + | ===Physiological Function of β-LG=== | |
| + | |||
| + | <StructureSection load='2gj5' size='280' side='right' caption='Structure of Beta Lactoglobulin with Vitamin D3 (PDB entry [[2gj5]])' scene=''> | ||
| + | <StructureSection load='1bso' size='280' side='right' caption='Structure of Beta Lactoglobulin with 12-BROMODODECANOIC ACID (PDB entry [[1bso]])' scene=''> | ||
| + | |||
| + | While no definite physiological function has been attributed to β-LG, several possibilities do exist. As a lipocalin it only stands to reason that most suggestions involve a role in molecular transport of small hydrophobic ligand transport (Kontopidis et al., 2002). Most research concentrates on the lactating cell or the neonate. β-Lactoglobulin does bind to fatty acids of intermediate to long length and retinol. It is very similar in structure to plasma retinol binding protein. Genetic variation does throw some doubt on these suggestions however, as β-Lactoglobulin in some species do not bind with fatty acids (Perez et al., 1989, 1993). This would suggest that its main function is in fact, not as a fatty acid transporter. It also seems unnecessary as a retinol binding transporting seeing as retinol associates more strongly with the fat in milk and does not depend on β-LG as its primary transporter. Data on β-LG’s role as an activity-modulator tends to be circumstantial. It has been suggested that the function of β-LG is directly related to maternal physiology (Kontopidis et al., 2002). | ||
| + | Nutritional value seems to be a valid purpose for β-LG. In the case of many species, the gene determining β-LG production has undergone a gene duplication and is involved more with nutrition than other functional purposes. Some species, like rodents, humans, and lagomorphs lost the ability to produce β-LG through formation of a pseudo-gene. While ligand binding and even the inhibition of harmful bacterial adhesion in the intestine (Ouwehand et al., 1997) are certainly useful properties, but neonatal nutrition continues to be the primary function. | ||
| + | |||
| + | |||
| + | </StructureSection> | ||
| + | |||
| + | ===Tanford Transition of β-LG=== | ||
| + | |||
| + | <StructureSection load='3blg' size='280' side='right' caption='Structure of Beta Lactoglobulin at pH 6.2 (PDB entry [[3blg]])' scene=''> | ||
| + | <StructureSection load='1bsy' size='280' side='right' caption='Structure of Beta Lactoglobulin at pH 7.1 (PDB entry [[1bsy]])' scene=''> | ||
| + | <StructureSection load='2blg' size='280' side='right' caption='Structure of Beta Lactoglobulin at pH 8.2 (PDB entry [[2blg]])' scene=''> | ||
| + | |||
| + | The structures of the trigonal crystal form of bovine beta-lactoglobulin variant A at pH 6.2, 7.1, and 8.2 have been determined by X-ray diffraction methods at a resolution of 2.56, 2. 24, and 2.49 A, respectively. The corresponding values for R (Rfree) are 0.192 (0.240), 0.234 (0.279), and 0.232 (0.277). The C and N termini as well as two disulfide bonds are clearly defined in these models. The glutamate side chain of residue 89 is buried at pH 6.2 and becomes exposed at pH 7.1 and 8.2. This conformational change, involving the loop 85-90, provides a structural basis for a variety of pH-dependent chemical, physical, and spectroscopic phenomena, known as the Tanford transition. | ||
| + | |||
| + | </StructureSection> | ||
| + | |||
| + | ===Implications=== | ||
| + | |||
| + | For the dairy industry, functionality and nutritional value are the primary issues when dealing with dairy proteins such as beta-lactoglobulin. The genetic variants referred to above in the ruminant species result in relatively minor amino acid differences, but the processing properties appear to be significantly affected even by these small changes, such that consideration has been given to removing the less favorable variants by selective breeding (Hill et al., 1997; Harris, 1997, Kontopidis et al., 2004). | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | |||
| + | G. Kontopidis, C. Holt, L. Sawyer. The ligand binding site of bovine β-lactoglobulin—Evidence for a function? J. Mol. Biol., 318 (2002), pp. 1043–1055 | ||
| + | |||
| + | J.P. Hill, M. Boland, A.F. Smith. The effect of β-lactoglobulin variants on milk powder manufacture and properties | ||
| + | J.P. Hill, M. Boland (Eds.), Milk Protein Polymorphism, International Dairy Federation, Brussels, Belgium (1997), pp. 372–394 | ||
| + | |||
| + | B.L. Harris. Selection to increase β-lactoglobulin B variant in New Zealand dairy cattle. J.P. Hill, M. Boland (Eds.), Milk Protein Polymorphism, International Dairy Federation, Brussels, Belgium (1997), pp. 467–473 | ||
| + | |||
| + | P. Havea, H. Singh, L.K. Creamer. Characterization of heat-induced aggregates of β-lactoglobulin, α-lactalbumin, and bovine serum albumin in a whey protein concentrate environment. J. Dairy Res., 68 (2001), pp. 483–497 | ||
| + | |||
| + | D.R. Flower, A.C.T. North, C.E. Sansom | ||
| + | The lipocalin protein family—structural and sequence overview. Biochim. Biophys. Acta, 1482 (2000), pp. 9–24 | ||
| + | |||
| + | A.C. Ouwehand, S.J. Salminen, M. Skurnik, P.L. Conway | ||
| + | Inhibition of pathogen adhesion by beta-lactoglobulin. Int. Dairy J., 7 (1997), pp. 685–692 | ||
| + | |||
| + | J.M.A. Tilley. The chemical and physical properties of bovine β-lactoglobulin | ||
| + | Dairy Sci. Abstr., 22 (1960), pp. 111–125 | ||
| + | |||
| + | R.L.J. Lyster. Reviews of the progress of dairy science. Section C. Chemistry of milk proteins. J. Dairy Res., 39 (1972), pp. 279–318 | ||
| + | |||
| + | J.E. Kinsella, D.M. Whitehead. Proteins in whey: Chemical, physical and functional properties. Adv. Food Nutr. Res., 33 (1989), pp. 343–438 | ||
| - | + | S.G. Hambling, A.S. McAlpine, L. Sawyer. β-Lactoglobulin. P.F. Fox (Ed.), Advanced Dairy Chemistry-I: Proteins, Elsevier, London, UK (1992), pp. 140–191 | |
| - | + | L. Sawyer. β-Lactoglobulin. P.F. Fox, P. McSweeney (Eds.), Advanced Dairy Chemistry I (3rd ed.), Kluwer, Amsterdam, The Netherlands (2003), pp. 319–386 | |
Current revision
Contents |
Beta Lactoglobulin
β-Lactoglobulin(β-LG) is the bulk constituent of whey protein in most ruminant species and is also present in the milks of most mammalian species, although not in rodent, human, and lagomorph milks. It has been studied extensively for some time ( Tilley, 1960; Lyster, 1972; Kinsella and Whitehead, 1987; Hambling et al., 1992; Sawyer, 2003). β-LG is a lipocalin as is seen through its amino-acid sequence and structure (Flower et al., 2000). Lipocalins are a varied family which bind small hydrophobic ligands, and often act as transporters in vivo. The purpose of β-LG, however, is not clear. Genetic variants of β-LG expressing genes have been reported, especially in cows and several nonruminant species (Kontopidis, 2004).
Whey is an often used food ingredient in the food industry and has many useful functional characteristics including foaming, gelling, thermal stability, emulsification, and protein fortification. It has also been suggested that it has many useful health benefits including exercise recovery, weight management, cardiovascular health, anti-cancer effects, anti-infection activity, wound repair, and infant nutrition. Human consumption of whey ingredients has steadily increased and has become more and more important to the dairy industry. An issue with whey ingredients is the subtle off flavor often associated with whey proteins. During the Cheddar cheese making process, norbixin leaches into the whey during the draining of the curd. Norbixin, a carotenoid extracted from the bixa orellana seed and added to cheese milk to give Cheddar its expected yellow flavor, would also contribute yellow color to the final product into which the whey ingredient is added. To prevent discoloration of the final product, whey is bleached by either hydrogen peroxide or benzoyl peroxide. In addition to a white color, this also causes lipid oxidation and associated off flavors.
| |||||||||||
Physiological Function of β-LG
| |||||||||||
Tanford Transition of β-LG
| |||||||||||
Implications
For the dairy industry, functionality and nutritional value are the primary issues when dealing with dairy proteins such as beta-lactoglobulin. The genetic variants referred to above in the ruminant species result in relatively minor amino acid differences, but the processing properties appear to be significantly affected even by these small changes, such that consideration has been given to removing the less favorable variants by selective breeding (Hill et al., 1997; Harris, 1997, Kontopidis et al., 2004).
References
G. Kontopidis, C. Holt, L. Sawyer. The ligand binding site of bovine β-lactoglobulin—Evidence for a function? J. Mol. Biol., 318 (2002), pp. 1043–1055
J.P. Hill, M. Boland, A.F. Smith. The effect of β-lactoglobulin variants on milk powder manufacture and properties J.P. Hill, M. Boland (Eds.), Milk Protein Polymorphism, International Dairy Federation, Brussels, Belgium (1997), pp. 372–394
B.L. Harris. Selection to increase β-lactoglobulin B variant in New Zealand dairy cattle. J.P. Hill, M. Boland (Eds.), Milk Protein Polymorphism, International Dairy Federation, Brussels, Belgium (1997), pp. 467–473
P. Havea, H. Singh, L.K. Creamer. Characterization of heat-induced aggregates of β-lactoglobulin, α-lactalbumin, and bovine serum albumin in a whey protein concentrate environment. J. Dairy Res., 68 (2001), pp. 483–497
D.R. Flower, A.C.T. North, C.E. Sansom The lipocalin protein family—structural and sequence overview. Biochim. Biophys. Acta, 1482 (2000), pp. 9–24
A.C. Ouwehand, S.J. Salminen, M. Skurnik, P.L. Conway Inhibition of pathogen adhesion by beta-lactoglobulin. Int. Dairy J., 7 (1997), pp. 685–692
J.M.A. Tilley. The chemical and physical properties of bovine β-lactoglobulin Dairy Sci. Abstr., 22 (1960), pp. 111–125
R.L.J. Lyster. Reviews of the progress of dairy science. Section C. Chemistry of milk proteins. J. Dairy Res., 39 (1972), pp. 279–318
J.E. Kinsella, D.M. Whitehead. Proteins in whey: Chemical, physical and functional properties. Adv. Food Nutr. Res., 33 (1989), pp. 343–438
S.G. Hambling, A.S. McAlpine, L. Sawyer. β-Lactoglobulin. P.F. Fox (Ed.), Advanced Dairy Chemistry-I: Proteins, Elsevier, London, UK (1992), pp. 140–191
L. Sawyer. β-Lactoglobulin. P.F. Fox, P. McSweeney (Eds.), Advanced Dairy Chemistry I (3rd ed.), Kluwer, Amsterdam, The Netherlands (2003), pp. 319–386