User:Eran Hodis/Sandbox7
From Proteopedia
(→Medical Implications) |
(→Medical Implications) |
||
| Line 39: | Line 39: | ||
Elevated cholesterol levels have been identified as a major risk factor for coronary artery disease, the narrowing of arteries of the heart, which affected over 13 million people in the United States alone. It is a major cause of disability and death, killing over 500 thousand people in the USA in 2001. <ref>www.nhlbi.nih.gov/health/.../Diseases/.../CAD_WhatIs.html</ref> | Elevated cholesterol levels have been identified as a major risk factor for coronary artery disease, the narrowing of arteries of the heart, which affected over 13 million people in the United States alone. It is a major cause of disability and death, killing over 500 thousand people in the USA in 2001. <ref>www.nhlbi.nih.gov/health/.../Diseases/.../CAD_WhatIs.html</ref> | ||
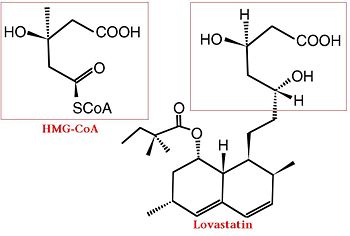
| - | The statins are HMG-CoA reductase inhibitors. Discovered by Akira Endo in 1971 | + | The statins are HMG-CoA reductase inhibitors. Discovered by Akira Endo in 1971, statins are similar in structure to HMG-CoA and act by competitively inhibiting HMGR. Since HMGR is the first committed enzyme in the cascade that eventually produces cholesterol, use of statins can dramatically reduce blood cholesterol levels.<ref>PMID:16386050</ref> As a drug class, statins generated over $20 billion dollars in sales in 2009 with Pfizer’s Lipitor being the best selling drug in the world at the time of writing.<ref>http://www.drugs.com/top200.html</ref><ref> http://www.medicalnewstoday.com/articles/25046.php</ref> |
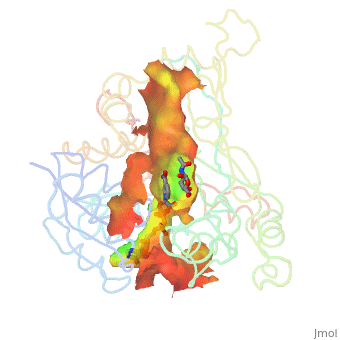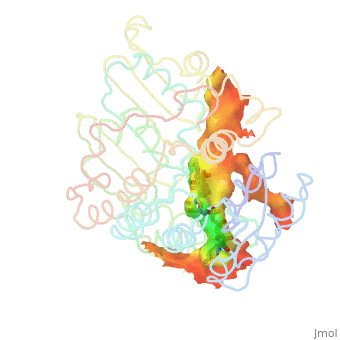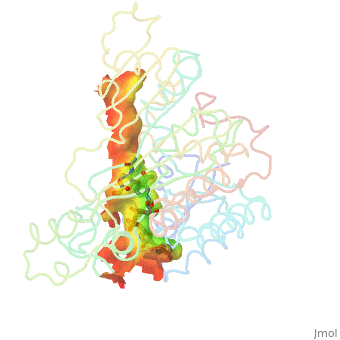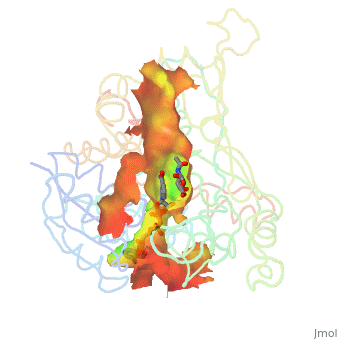
A number of crystal structures of HMGR with bound statins have been solved which elucidate how the statin molecule is bound by HMGR. Statins in general occupy the active site of HMGR, preventing HMG-CoA from binding. The structure of HMGR with bound statins (<scene name='HMG-CoA_Reductase/Statin_ator/3'>here atorvastatin, marketed as Lipitor</scene>) shows that the cis loop forms a number of polar interactions with the statin inhibitor, particularly residues Ser 684, Asp 690, Lys 691, Lys 692, and hydrogen bond interactions between Glu 559 and Asp 767 with the O5-hydroxyl of the statins. Van der Waals interactions between Leu 562, Val 683, Leu 853, Ala 856, and Leu 857 of HMGR and hydrophobic ring structures of the statins contribute to binding as well.<ref>PMID:11349148</ref>These interactions result in the statins binding to HMGR with a K<sub>i</sub> of between .1-2.3nM while the Michaelis constant K<sub>M</sub> for HMG-CoA is 4uM, allowing the statins to outcompete HMG-CoA in binding to HMGR.<ref>PMID:7784310</ref> Additional structures of HMGR with the statins <scene name='HMG-CoA_Reductase/Statin_meva/3'>mevastatin</scene>, <scene name='HMG-CoA_Reductase/Statin_simva/3'>simvastatin (Zocor)</scene>, <scene name='HMG-CoA_Reductase/Statin_fluva/4'>fluvastatin (Lescol)</scene>, <scene name='HMG-CoA_Reductase/Statin_ceriv/2'>cerivastatin (Baycol)</scene>, and <scene name='HMG-CoA_Reductase/Statin_rosu/4'>rosuvastatin (Crestor)</scene> highlight the important residues involved in inhibitor binding. | A number of crystal structures of HMGR with bound statins have been solved which elucidate how the statin molecule is bound by HMGR. Statins in general occupy the active site of HMGR, preventing HMG-CoA from binding. The structure of HMGR with bound statins (<scene name='HMG-CoA_Reductase/Statin_ator/3'>here atorvastatin, marketed as Lipitor</scene>) shows that the cis loop forms a number of polar interactions with the statin inhibitor, particularly residues Ser 684, Asp 690, Lys 691, Lys 692, and hydrogen bond interactions between Glu 559 and Asp 767 with the O5-hydroxyl of the statins. Van der Waals interactions between Leu 562, Val 683, Leu 853, Ala 856, and Leu 857 of HMGR and hydrophobic ring structures of the statins contribute to binding as well.<ref>PMID:11349148</ref>These interactions result in the statins binding to HMGR with a K<sub>i</sub> of between .1-2.3nM while the Michaelis constant K<sub>M</sub> for HMG-CoA is 4uM, allowing the statins to outcompete HMG-CoA in binding to HMGR.<ref>PMID:7784310</ref> Additional structures of HMGR with the statins <scene name='HMG-CoA_Reductase/Statin_meva/3'>mevastatin</scene>, <scene name='HMG-CoA_Reductase/Statin_simva/3'>simvastatin (Zocor)</scene>, <scene name='HMG-CoA_Reductase/Statin_fluva/4'>fluvastatin (Lescol)</scene>, <scene name='HMG-CoA_Reductase/Statin_ceriv/2'>cerivastatin (Baycol)</scene>, and <scene name='HMG-CoA_Reductase/Statin_rosu/4'>rosuvastatin (Crestor)</scene> highlight the important residues involved in inhibitor binding. | ||
Revision as of 14:47, 31 October 2010
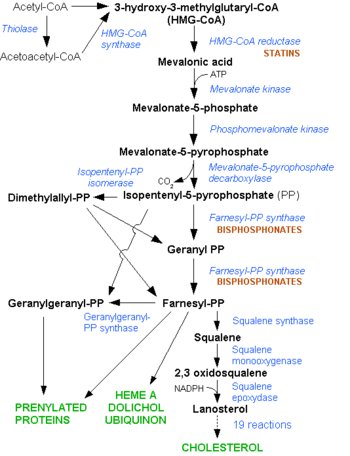
HMG-CoA Reductase (or 3-hydroxy-3-methyl-glutaryl-CoA reductasese or HMGR) is the rate-controlling enzyme of the metabolic pathway responsible for cholesterol and other isoprenoid biosynthesis, the mevalonate pathway. HMGR is a polytopic, transmembrane protein, containing 8 domains, that is anchored in the membrane of the endoplasmic reticulum.[1] It is the major target of the statins, a cholesterol lowering drug class and the best selling pharmaceutical drugs in the world.
Contents
|
Biological Role
HMGR is among the most highly regulated enzymes in the human body. It catalyzes the formation of mevalonic acid, the committed step in the biosynthesis of sterols, notably cholesterol and isoprenoids. This reaction can be seen below where HMG-CoA is reduced by NADPH. Despite the poor reputation cholesterol has in the media, it is a critical component of cellular membranes as it is required to establish proper membrane permeability and fluidity. The mevalonate pathway is also responsible for synthesis of the oxygen transporting heme found in red blood cells. [2]
Structure
|
|
Regulation of HMG-CoA Reductase
As stated before, HMGR is among the most highly regulated enzymes in the human body. [2] Regulation of HMG-CoA reductase occurs at 4 different levels of feedback regulation. First by regulation of transcription of the reductase gene, which is activated by sterol regulatory element binding protein, a protein that binds to the promoter of the HMGR gene when cholesterol levels fall. The second level of regulation is at the translation of the HMGR mRNA, which is inhibited by Farnesol, a derivative of the mevalonate pathway. [2]
The third level of HMGR regulation involves the degradation of intact reductase. As the level of sterols increases, HMGR reductase becomes more susceptible to ER-associated degradation. Helices 2-6 of the HMGR transmembrane domain, called the sterol-sensing domain, sense the increased levels of sterols. Degradation of HMGR is initiated when a membrane-bound cysteine protease cleaves within the transmembrane region of HMGR. Additionally, as sterol levels increase, the helices can slightly rearrange, exposing Lysine 248 which can be ubiquitinated and subsequently trigger proteolytic degradation. [1] [3]
A final level of HMGR regulation is achieved by inhibition via . HMGR is phosphorylated and inactivated by an AMP-activated protein kinase, when the energy charge of the cell is low and AMP concentrations are high. Since Serine 872 is in the vicinity of the pohsphate of NADP, phosphorylation likely results in a decreased affinity for NADPH, halting the formation of Mevaldyl-CoA from HMG-CoA. [4]
Medical Implications
|
Elevated cholesterol levels have been identified as a major risk factor for coronary artery disease, the narrowing of arteries of the heart, which affected over 13 million people in the United States alone. It is a major cause of disability and death, killing over 500 thousand people in the USA in 2001. [5]
The statins are HMG-CoA reductase inhibitors. Discovered by Akira Endo in 1971, statins are similar in structure to HMG-CoA and act by competitively inhibiting HMGR. Since HMGR is the first committed enzyme in the cascade that eventually produces cholesterol, use of statins can dramatically reduce blood cholesterol levels.[6] As a drug class, statins generated over $20 billion dollars in sales in 2009 with Pfizer’s Lipitor being the best selling drug in the world at the time of writing.[7][8]
A number of crystal structures of HMGR with bound statins have been solved which elucidate how the statin molecule is bound by HMGR. Statins in general occupy the active site of HMGR, preventing HMG-CoA from binding. The structure of HMGR with bound statins () shows that the cis loop forms a number of polar interactions with the statin inhibitor, particularly residues Ser 684, Asp 690, Lys 691, Lys 692, and hydrogen bond interactions between Glu 559 and Asp 767 with the O5-hydroxyl of the statins. Van der Waals interactions between Leu 562, Val 683, Leu 853, Ala 856, and Leu 857 of HMGR and hydrophobic ring structures of the statins contribute to binding as well.[9]These interactions result in the statins binding to HMGR with a Ki of between .1-2.3nM while the Michaelis constant KM for HMG-CoA is 4uM, allowing the statins to outcompete HMG-CoA in binding to HMGR.[10] Additional structures of HMGR with the statins , , , , and highlight the important residues involved in inhibitor binding.
Additional 3D Structures of HMG-CoA Reductase
3cct, 3ccw, 3ccz, 3cd0, 3cd5, 3cd7, 3cda, 3cdb – HMG-CoA Reductase Catalytic Domain
2qil – HMG-CoA Reductase Catalytic Domain
2q6b, 2q6c – Catalytic Domain of HMG-CoA Reductase
1t02 – Crystal Structure of Statin Bound HMG-CoA Reductase
1r31 – Crystal Structure of HMGR from Pseudomonas mevalonii
1hw8, 1hw9, 1hwi, 1hwj, 1hwk, 1hwl – HMG CoA Reductase with Various Statins Bound
1qax, 1qay – Ternary Complex of Pseudomonas Mevalonii HMGR with mevalonate
Additional Resources
- See: Pharmaceutical Drug Targets For Additional Information about Drug Targets for Related Diseases
- See: Metabolic Disorders For Additional Information.
References
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedRoitelman - ↑ 2.0 2.1 2.2 Meigs TE, Roseman DS, Simoni RD. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase degradation by the nonsterol mevalonate metabolite farnesol in vivo. J Biol Chem. 1996 Apr 5;271(14):7916-22. PMID:8626470
- ↑ Song BL, Sever N, DeBose-Boyd RA. Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol Cell. 2005 Sep 16;19(6):829-40. PMID:16168377 doi:10.1016/j.molcel.2005.08.009
- ↑ Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425-30. PMID:1967820 doi:http://dx.doi.org/10.1038/343425a0
- ↑ www.nhlbi.nih.gov/health/.../Diseases/.../CAD_WhatIs.html
- ↑ Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323-6. PMID:16386050
- ↑ http://www.drugs.com/top200.html
- ↑ http://www.medicalnewstoday.com/articles/25046.php
- ↑ Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001 May 11;292(5519):1160-4. PMID:11349148 doi:10.1126/science.1059344
- ↑ Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 1995 Jan;31(1):9-27. PMID:7784310