We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Shank protein
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
__TOC__ | __TOC__ | ||
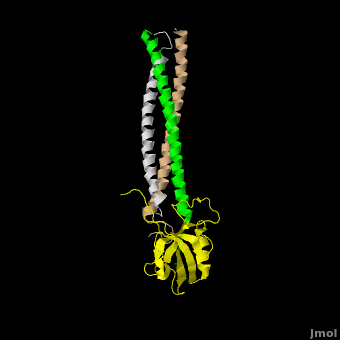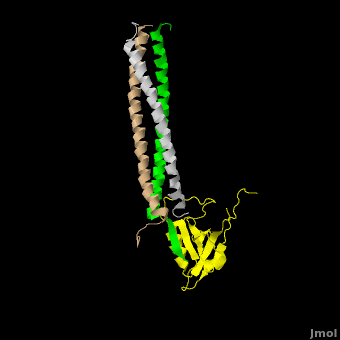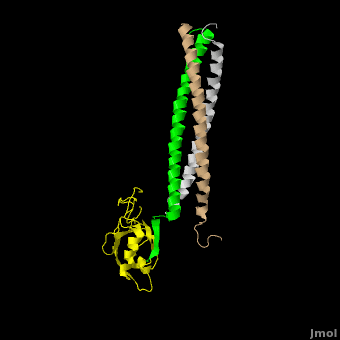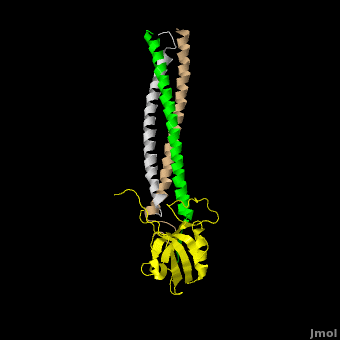
[[Image:Shank Schematic.png|150px|left]] '''Shank (SH3 and multiple ankyrin repeat domains protein) Family Proteins''' are scaffolding proteins found in the postsynaptic density (PSD) of excitatory synapses. The PSD, a structure within the postsynaptic membrane of dendritic spines, contains a complex assembly of proteins which organize neurotransmitter receptors and regulatory elements.<ref name="Park">PMID:12626503</ref> The PSD coordinates communication of incoming signals to various targets and changes its composition in response to neural signals to aid neuronal plasticity<ref name="Baron">PMID:16439662</ref> Shank proteins function as the master organizer of the PSD with their ability to recruit and form multimeric complexes with postsynaptic receptors, signaling molecules, and cytoskeletal proteins, like AMPA, [[Neuroligin-Neurexin Interaction|Neuroligin]] and NMDA [[Glutamate Receptors|glutamate receptors]].<ref name="Durand">PMID:17173049</ref> Within the PSD, there are over 300 individual shank molecules, roughly 5% of the total protein molecules within the PSD.<ref name="Bozdagi">PMID: 21167025</ref> Shanks contain five domains for protein-protein interactions, including an ankyrin repeat domain, used to bind acting regulating proteins, an Src homology 3 (Sh3) domain, used to bind AMPA receptors, a PDZ domain, used to bind G protein coupled receptors, several proline-rich domains, and a C-terminal SAM domain, which is responsible for mediating Shank multimerization. (See Image)<ref name="Park"/> Shank also mediates the maturation of dendritic spines in neurons.<ref name="Durand"/> See also [[Neurodevelopmental Disorders]]. | [[Image:Shank Schematic.png|150px|left]] '''Shank (SH3 and multiple ankyrin repeat domains protein) Family Proteins''' are scaffolding proteins found in the postsynaptic density (PSD) of excitatory synapses. The PSD, a structure within the postsynaptic membrane of dendritic spines, contains a complex assembly of proteins which organize neurotransmitter receptors and regulatory elements.<ref name="Park">PMID:12626503</ref> The PSD coordinates communication of incoming signals to various targets and changes its composition in response to neural signals to aid neuronal plasticity<ref name="Baron">PMID:16439662</ref> Shank proteins function as the master organizer of the PSD with their ability to recruit and form multimeric complexes with postsynaptic receptors, signaling molecules, and cytoskeletal proteins, like AMPA, [[Neuroligin-Neurexin Interaction|Neuroligin]] and NMDA [[Glutamate Receptors|glutamate receptors]].<ref name="Durand">PMID:17173049</ref> Within the PSD, there are over 300 individual shank molecules, roughly 5% of the total protein molecules within the PSD.<ref name="Bozdagi">PMID: 21167025</ref> Shanks contain five domains for protein-protein interactions, including an ankyrin repeat domain, used to bind acting regulating proteins, an Src homology 3 (Sh3) domain, used to bind AMPA receptors, a PDZ domain, used to bind G protein coupled receptors, several proline-rich domains, and a C-terminal SAM domain, which is responsible for mediating Shank multimerization. (See Image)<ref name="Park"/> Shank also mediates the maturation of dendritic spines in neurons.<ref name="Durand"/> See also [[Neurodevelopmental Disorders]]. | ||
| + | *'''SHANK1''' mutations were detected in individuals with autism spectrum disorders<ref>PMID:26335738</ref>. | ||
*'''SHANK2''' is located at the postsynaptic membrane of glutamatergic neurons<ref>PMID:32987185</ref>. | *'''SHANK2''' is located at the postsynaptic membrane of glutamatergic neurons<ref>PMID:32987185</ref>. | ||
*'''SHANK3''' is enriched at the postsynaptic density of excitatory synapses<ref>PMID:22749736</ref>. | *'''SHANK3''' is enriched at the postsynaptic density of excitatory synapses<ref>PMID:22749736</ref>. | ||
Current revision
| |||||||||||
- ↑ 1.0 1.1 1.2 Park E, Na M, Choi J, Kim S, Lee JR, Yoon J, Park D, Sheng M, Kim E. The Shank family of postsynaptic density proteins interacts with and promotes synaptic accumulation of the beta PIX guanine nucleotide exchange factor for Rac1 and Cdc42. J Biol Chem. 2003 May 23;278(21):19220-9. Epub 2003 Mar 7. PMID:12626503 doi:10.1074/jbc.M301052200
- ↑ 2.0 2.1 2.2 Baron MK, Boeckers TM, Vaida B, Faham S, Gingery M, Sawaya MR, Salyer D, Gundelfinger ED, Bowie JU. An architectural framework that may lie at the core of the postsynaptic density. Science. 2006 Jan 27;311(5760):531-5. PMID:16439662 doi:311/5760/531
- ↑ 3.0 3.1 3.2 Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007 Jan;39(1):25-7. Epub 2006 Dec 17. PMID:17173049 doi:ng1933
- ↑ 4.0 4.1 4.2 4.3 Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010 Dec 17;1(1):15. PMID:21167025 doi:10.1186/2040-2392-1-15
- ↑ Gong X, Wang H. SHANK1 and autism spectrum disorders. Sci China Life Sci. 2015 Oct;58(10):985-90. PMID:26335738 doi:10.1007/s11427-015-4892-6
- ↑ Caumes R, Smol T, Thuillier C, Balerdi M, Lestienne-Roche C, Manouvrier-Hanu S, Ghoumid J. Phenotypic spectrum of SHANK2-related neurodevelopmental disorder. Eur J Med Genet. 2020 Dec;63(12):104072. PMID:32987185 doi:10.1016/j.ejmg.2020.104072
- ↑ Uchino S, Waga C. SHANK3 as an autism spectrum disorder-associated gene. Brain Dev. 2013 Feb;35(2):106-10. PMID:22749736 doi:10.1016/j.braindev.2012.05.013
- ↑ Garber K. Neuroscience. Autism's cause may reside in abnormalities at the synapse. Science. 2007 Jul 13;317(5835):190-1. PMID:17626859 doi:10.1126/science.317.5835.190
- ↑ Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, Lao K, Kosik KS. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008 Jul;9(3):153-61. Epub 2008 Jun 19. PMID:18563458 doi:10.1007/s10048-008-0133-5
- ↑ 10.0 10.1 10.2 10.3 Im YJ, Kang GB, Lee JH, Park KR, Song HE, Kim E, Song WK, Park D, Eom SH. Structural basis for asymmetric association of the betaPIX coiled coil and shank PDZ. J Mol Biol. 2010 Mar 26;397(2):457-66. Epub 2010 Jan 29. PMID:20117114 doi:10.1016/j.jmb.2010.01.048
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Alexander Berchansky, Joel L. Sussman