User:Laura Carbone/Sandbox 1
From Proteopedia
| Line 6: | Line 6: | ||
Shimonmura was originally looking only to isolate the blue luminescent protein of Aequorea victoria, traditionally thought to be luciferase, but it would soon become apparent that the glow was in fact due to aequorin, a substance related, but slightly varying from luciferase. <ref name="Shimonmura"><ref name="Haldar"> However, the light emitted from aequorin still differed from the light emitted from the wild jellyfish. This quandary led to the discovery of the green fluorescent protein responsible for this disparity, but sufficient amounts of the protein could not be collected for study until 1979. The journey to discover the nature of GFP had begun.<ref name="Shimonmura"> | Shimonmura was originally looking only to isolate the blue luminescent protein of Aequorea victoria, traditionally thought to be luciferase, but it would soon become apparent that the glow was in fact due to aequorin, a substance related, but slightly varying from luciferase. <ref name="Shimonmura"><ref name="Haldar"> However, the light emitted from aequorin still differed from the light emitted from the wild jellyfish. This quandary led to the discovery of the green fluorescent protein responsible for this disparity, but sufficient amounts of the protein could not be collected for study until 1979. The journey to discover the nature of GFP had begun.<ref name="Shimonmura"> | ||
| + | |||
| + | In the 1990’s, Douglas Prasher, Frankly Predergast, and co-workers successfully cloned the gene that encoded for GFP. Martin Chalfie further pursued this line of work and was eventually able to express GFP in heterologous systems such as E. coli and C. elegans. Chalfie’s research provided the first evidence that GFP was unique as it did not require the presence of any exogenous substance or cofactor for fluorescence (Haldar & Chattopadhyay, 2009). | ||
| + | |||
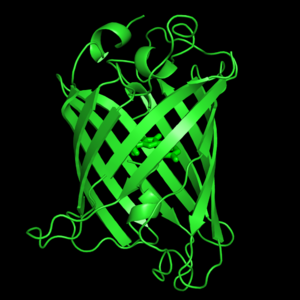
| + | Roger Tsien and co-workers were intrigued by the absence of a necessary cofactor and began to research the structure of GFP and how it relates to its fluorescence. They discovered that a helix within the beta barrel structure of GFP actually contained a fluorophore responsible for fluorescence. In researching its structure, they were able to develop GFP derivatives with improved fluorescence and photo-stability. Shimonmura, Chalfie, and Tsien were each recognized for their work involving GFP with the Nobel Prize in 2008 (Haldar & Chattopadhyay, 2009). In the time since the work of these three researchers, GFP has been successfully expressed and utilized in bacteria, yeast, slime mold, plants, drosophila fruit flies, zebra-fish, and mammalian cells (Yang et al., 1996). | ||
| + | |||
| + | Green fluorescent protein has had great success as a marker protein in a variety of biological systems due to its inherent stability, which only adds to its many other desirable characteristics as a marker proteins (Haldar & Chattopadhyay, 2009). GFP is rather resistant to denaturation (Yang et al., 1996), sustaining structure and function up to 65°C, pH 11, 1% sodium dodecyl sulphate (SDS), or 6 M guanidinium chloride. GFP can also withstand the presence of most proteases for many hours (Cubitt et al., 1995). Due to this stability, GFP could be applied to numerous other applications such as cell lineage tracing, gene expression reporting, or protein-protein interactions (Yang et al., 1996). | ||
{{Reflist}} | {{Reflist}} | ||
Revision as of 16:32, 2 February 2010
Green fluorescent protein (GFP) is a bioluminescent polypeptide consisting of 238 residues isolated from the body of Aequorea victoria jellyfish.[1] GFP converts the blue chemiluminescent of aequorin in the jellyfish into green fluorescent light.[2] In the laboratory, GFP can be incorporated into a variety of biological systems in order to function as a marker protein. Since its discovery in 1962, GFP has become a significant contributor to the research of monitoring gene expression, localization, mobility, traffic, interactions between various membrane and cytoplasmic proteins, as well as many others.[3]
Aequorea victoria was first discovered and investigated for its bioluminescence by Frank Johnson, who invited Osamu Shimonmura to work with him in on a small island not far from British Columbia, where the jellyfish is abundant.[4] Found off the west coast of the United States between British Columbia and central California (Cowles & Cowles, 2007), the jellyfish was considered a local phenomenon as it would drift in and out of the harbors.[4]