User:Laura Carbone/Sandbox 1
From Proteopedia
| Line 12: | Line 12: | ||
Green fluorescent protein has had great success as a marker protein in a variety of biological systems due to its inherent stability, which only adds to its many other desirable characteristics as a marker proteins.<ref name="Haldar"> GFP is rather resistant to denaturation,<ref name="Yang"> sustaining structure and function up to 65°C, pH 11, 1% sodium dodecyl sulphate (SDS), or 6 M guanidinium chloride. GFP can also withstand the presence of most proteases for many hours.<ref name="Cubitt">[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TCV-40W0TN7-50&_user=4187488&_coverDate=11%2F30%2F1995&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000062504&_version=1&_urlVersion=0&_userid=4187488&md5=e92730038bb92b1dfbd4af45a0283cce],Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien R. 1995. Understanding, improving, and using green fluorescent protein. Trends in Biochemical Sciences. 20(11): 448-455. DOI 0.1016/S0968-0004(00)89099-4.</ref> Due to this stability, GFP could be applied to numerous other applications such as cell lineage tracing, gene expression reporting, or protein-protein interactions.<ref name="Yang"> | Green fluorescent protein has had great success as a marker protein in a variety of biological systems due to its inherent stability, which only adds to its many other desirable characteristics as a marker proteins.<ref name="Haldar"> GFP is rather resistant to denaturation,<ref name="Yang"> sustaining structure and function up to 65°C, pH 11, 1% sodium dodecyl sulphate (SDS), or 6 M guanidinium chloride. GFP can also withstand the presence of most proteases for many hours.<ref name="Cubitt">[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TCV-40W0TN7-50&_user=4187488&_coverDate=11%2F30%2F1995&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000062504&_version=1&_urlVersion=0&_userid=4187488&md5=e92730038bb92b1dfbd4af45a0283cce],Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien R. 1995. Understanding, improving, and using green fluorescent protein. Trends in Biochemical Sciences. 20(11): 448-455. DOI 0.1016/S0968-0004(00)89099-4.</ref> Due to this stability, GFP could be applied to numerous other applications such as cell lineage tracing, gene expression reporting, or protein-protein interactions.<ref name="Yang"> | ||
| + | |||
| + | While GFP can be incorporated into most prokaryotic systems, expression in eukaryotic systems may be limited to the cytoplasm and the nucleus, as GFP does not penetrate the nucleolus or vesicular organelles. However, highly specific intracellular localization can still be achieved in eukaryotes,<ref name="Yang"> which can help to avoid the difficulties associated with adding extrinsic dyes (Van Thor & Sage, 2006). | ||
| + | |||
| + | ==Structure== | ||
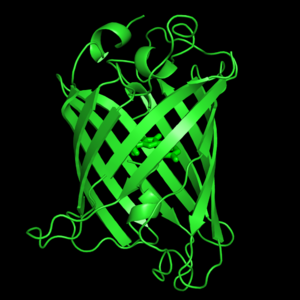
| + | Green fluorescent protein consists of 238 residues strung together to form five α-helices and one β-pleated sheet consisting of eleven strands (PDBsum, 2009), where each strand contains nine to thirteen residues each (Ormo, Cubitt, Kallio, Gross, & Tsien, 1996). These β-strands display an almost “seamless symmetry” in which only two of the strands vary in structural content (Phillips, 2007). This β-sheet conforms itself through regular hydrogen bonding into a β-barrel (Yang et al., 1996). In GFP, the structure is so regular that “stripes” of water molecules can be seen following the structure of the barrel (Phillips, 2007). Together with the α-helices at either end of the molecule, a nearly perfect cylinder is produced, 42Å long and 24Å in diameter (Ormo et al., 1996), creating what is referred to as a “β-can” formation (Phillips, 2007). The short helical segments at either end of the cylinder form “caps” to further protect the interior of the β-barrel (Phillips, 2007). Overall stability is maintained by this β-can structure, helping to resist unfolding from heat and other denaturants.<ref name="Yang"> | ||
| + | One α-helix can be found running through the central axis of the β-barrel (Haldar & Chattopadhyay, 2009), roughly perpendicular to the symmetry axis of the barrel (Ormo et al., 1996). This helix is extremely important as it contains the fluorophore responsible for fluorescence.<ref name="Haldar"><ref name="Yang"> This α-helix in particular is highly stabilized by the many contacts that are made with each strand of the barrel (Andrews, Gosavi, Finke, Onuchic, & Jennings, 2008). | ||
| + | The chromophore of GFP is located at the center of the β-barrel with a wild-type excitation peak of 395 nm, and a minor peak at 475 nm (Ormo et al., 1996; Phillips, 2007)<ref name="Yang"><ref name="Cubitt"> with extinction coefficients of approximately 30,000 and 7,000 M-1 cm-1, respectively (Yang et al., 1996; Phillips, 2007). However, the relative amplitudes of these two excitation peaks can vary depending on environmental factors and previous illumination (Ormo et al., 1996). For example, continued excitation leads to a diminution of the 395 nm excitation peak with a reciprocal amplification of the 475 nm peak (Phillips, 2007). Regardless of absorption, the chromophore of GFP emits light at 508 nm (Ormo et al., 1996; Phillips, 2007).<ref name="Yang"><ref name="Cubitt"> | ||
| + | Three amino residues in the central α-helix constitute the fluorophore of GFP: Ser65-Tyr66-Gly67. Tsien et al. discovered that this tri-peptide sequence is post-translationally modified by internal cyclization and oxidation (Haldar & Chattopadhyay, 2009) to produce a 4-(p-hydroxybenzylidene)-imidazolidin-5-one structure.<ref name="Yang"> Studies with E. coli proposed a sequential mechanism for the formation of the fluorophore that was initiated by a rapid cyclization between Ser65 and Gly67 to form an imidazolin-5-one intermediate.<ref name="Yang"> This rapid cyclization is carried out via nucleophilic attack of the amino group from Gly67 on the carbonyl group of Ser65 to form a five-membered ring. The loss of water then forms the imidazolin-5-one intermediate.<ref name="Cubitt"> Cyclization is succeeded by a much slower rate-limiting oxygenation of the Tyr66 hydroxybenzyl side chain by atmospheric oxygen (No fluorescence was seen in anaerobically grown E. coli.) (Phillips, 2007).<ref name="Yang"><ref name="Cubitt"> The double bond that results from this series of reactions results in the linkage of the two π-systems of the rings, forming a larger conjugated system essential for fluorophore stability (Bublitz, King, & Boxer, 1998; Lammich, Petersen, Nielsen, & Andersen, 2007). | ||
| + | The process is completely auto-catalytic such that there are no known co-factors or enzymatic components required (Yang et al., 1996). Despite the stability of the final product, while the chromophore is forming, the environmental temperature cannot drop below 30°C or the yield of viable GFP will decrease substantially (Phillips, 2007).<ref name="Yang"> This final stability is maintained through a network of close contacts surrounding the fluorophore.<ref name="Yang"> | ||
| + | As the central α-helix is not located directly in the center of the β-barrel, cavities of differing area exist on either side of the chromophore. The larger cavity, consisting of about 135 Å (Ormo et al., 1996), does not open out to the bulk solvent, but rather houses four water molecules (Ormo et al., 1996; Van Thor & Sage, 2006). Had this space not been occupied, it would be expected to considerably destabilize the protein as a whole. The hydrogen bonding created by the presence of the water molecules, however, helps to link the buried side chains of Glu222 and Gln69 that would otherwise be actively polar (Ormo et al., 1996). Therefore, the water molecules are extremely important in establishing a hydrogen bonding network about the chromophore (Lammich et al., 2007). | ||
| + | The opposite side of the chromophore, however, is within close proximity of several aromatic and polar side chains. Several polar interactions between the surrounding residues and the chromophore are present including: hydrogen bonds of His148, Thr203, and Ser205 with the phenolic hydroxyl of Tyr66; Arg96 and Gln94 with the carbonyl of the imidazolidinone ring; and hydrogen bonds of Glu222 with the side chain of Thr65. Additional hydrogen bonding in the area around the chromophore helps to stabilize Arg96 in the protonated form, which suggests the presence of a partial negative charge on the carbonyl oxygen of the imidazolidinone ring in the deprotonated fluorophore (Ormo et al., 1996). Arg96 and Gln94 in turn help to steady the imidazolidone.<ref name="Yang"> Therefore, it is thought that Arg96 is essential for the formation of the fluorophore by catalyzing the initial ring closure (Ormo et al., 1996). The only tryptophan in the whole GFP sequence is located 13 to 15 Å from the chromophore, providing a stabilizing edge-face interaction with the benzyl ring of the chromophore (Ormo et al., 1996). The stability provided by the internal polar interactions are further augmented by the surrounding β-barrel. | ||
| + | The β-barrel provides a highly constrained environment that protects the chromophore from the bulk solvent (Haldar & Chattopadhyay, 2009), nearly creating the atmosphere of a vacuum (Lammich et al., 2007). This is most likely responsible for the small Stoke’s shift, or the small wavelength difference between excitation and emission (Ormo et al., 1996). | ||
| + | Findings show that fluorescence will not occur from a naked chromophore, but rather requires the protection of the β-can structure.<ref name="Cubitt"> However, in crystallum GFP will exhibit a nearly identical fluorescence spectrum and lifetime when compared with aqueous GFP. These two elements point to a fluorescence that is not inherent to the isolated fluorophore (Phillips, 2007),<ref name="Yang"> but rather from the auto-catalytic cyclization of the polypeptide sequence Ser65Tyr66Gly67 and subsequent oxidation of Tyr66 (Phillips, 2007). However, this sequence is found in many proteins - why does GFP fluoresce? According to Phillips (1997), fluorescence is due to the close proximity of the backbone atoms between Ser65 and Gly67 gained through a lack of sterical hindrance by the hydrogen atom side chain of glycine. In fact, no functional fluorescent proteins have been found in which any other amino acid other than glycine was found at position 67. Even so, there are still proteins that have this specific sequence, therefore, there must be another inherent property to GFP that is still left misunderstood (Phillips, 2007). | ||
| + | This quandary led Phillips to study the acid/base chemistry catalyzing the initial cyclization of the chromophore. He found that Arg96 actually acts as a base by withdrawing electrons through hydrogen bonding with the carbonyl oxygen of Ser65 to activate the carbonyl carbon for nucleophilic attack by the amide nitrogen of Gly67. This mechanism was further supported by ab initio calculations, as well as database searches of similar compounds and protein sequences. Through acid/base chemistry, the chromophore is stabilized by resonance (Phillips, 2007). | ||
| + | Many mutant green fluorescent proteins have been developed in order to further understand the structure and mechanism of the fluorophore. The first mutagenesis studies simply truncated the ends of the amino acid sequence. Shortening the polypeptide by more than seven amino acids from either terminus lead to a total loss of fluorescence, as well as a complete failure to absorb light at the traditional wavelengths. This is most likely due to the structure of the protein. The last seven amino acid residues of the carboxyl terminus are roughly disordered, and thus do not interfere with the overall structure. After seven residues, however, the capping α-helix structure is disrupted, leading to an unstable or unformed chromophore. The amino terminus is less understood, but the same principle still applies even though the β-barrel does not begin until residue ten or eleven.<ref name="Yang"> | ||
| + | Point mutations have also been extensively studied in order to examine their effects on the chromophore. In general, most point mutations lead to a diminished excitation, especially in regions of the sequence adjacent to the fluorophore or those that interact with the fluorophore. An exception to this trend is the Ser65Thr66 mutant (normal Ser65Tyr66), which actually increases fluorescence intensity, although the reason is unclear.<ref name="Yang"> | ||
| + | An interesting mutation discovered by Ormo et al. (1996) was the Thr65Tyr66Gly67 mutant, which produces an α-helical conformation in the chromophore opposed to the normal conformation, which is nearly perpendicular to the helical axis, due to its interaction with Arg96. This further supports the idea that Arg96 is an important factor in the structural arrangement required for cyclization, perhaps by promoting the attack of Gly67 on the carbonyl carbon of Thr65 (Ormo et al., 1996). | ||
| + | In high protein concentrations, GFP has been found to dimerize under the influence of high ionic strength between the two monomers. In Aequorea victoria, the aequorin is able to bind to the dimer, but not the monomer. Therefore, dimerization is a very important structural feature in terms of its function, as it also assists the GFP to absorb energy at the excitation wavelength of aequorin even though GFP has only a “modest” extinction coefficient. As a result, dimers, and often even higher multimers, are predominant protein populations within the jellyfish.<ref name="Cubitt"> | ||
{{Reflist}} | {{Reflist}} | ||
Revision as of 02:46, 3 February 2010
Green fluorescent protein (GFP) is a bioluminescent polypeptide consisting of 238 residues isolated from the body of Aequorea victoria jellyfish.[1] GFP converts the blue chemiluminescent of aequorin in the jellyfish into green fluorescent light.[2] In the laboratory, GFP can be incorporated into a variety of biological systems in order to function as a marker protein. Since its discovery in 1962, GFP has become a significant contributor to the research of monitoring gene expression, localization, mobility, traffic, interactions between various membrane and cytoplasmic proteins, as well as many others.[3]
Aequorea victoria was first discovered and investigated for its bioluminescence by Frank Johnson, who invited Osamu Shimonmura to work with him in on a small island not far from British Columbia, where the jellyfish is abundant.[4] Found off the west coast of the United States between British Columbia and central California,[5] the jellyfish was considered a local phenomenon as it would drift in and out of the harbors.[4] Due to this stability, GFP could be applied to numerous other applications such as cell lineage tracing, gene expression reporting, or protein-protein interactions.[2]