This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Amyloid beta
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
The first 16 residues, Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-Tyr-Glu--Val-His-His-Gln-Lys, are mostly hydrophobic with <scene name='Amyloid_beta/Cu/1'>His13 and His14</scene> form a binding domain for Cu(II). Residues <scene name='Amyloid_beta/Self/2'>12-23</scene> function as the self recognition region allowing for the formation of dimers and/or oligomers. This region also serves as the binding site for cholesterol, apolipoproteinE, alpha7nAChr, and amyloid beta-peptide binding alcohol dehydrogenase.<ref name="alz" /> | The first 16 residues, Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-Tyr-Glu--Val-His-His-Gln-Lys, are mostly hydrophobic with <scene name='Amyloid_beta/Cu/1'>His13 and His14</scene> form a binding domain for Cu(II). Residues <scene name='Amyloid_beta/Self/2'>12-23</scene> function as the self recognition region allowing for the formation of dimers and/or oligomers. This region also serves as the binding site for cholesterol, apolipoproteinE, alpha7nAChr, and amyloid beta-peptide binding alcohol dehydrogenase.<ref name="alz" /> | ||
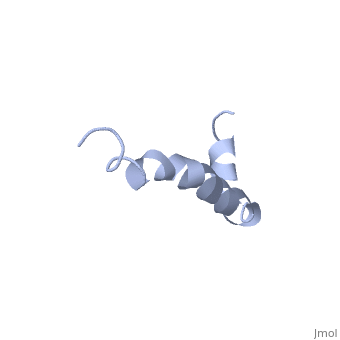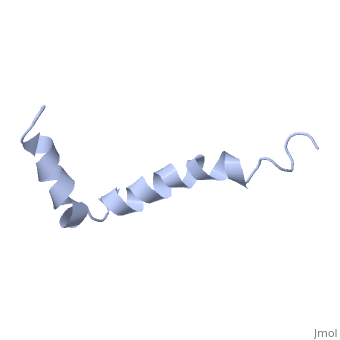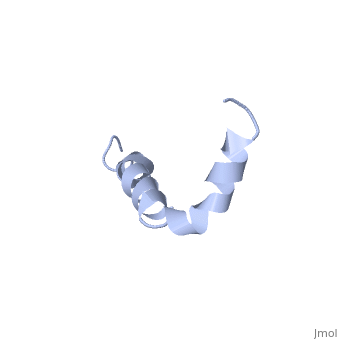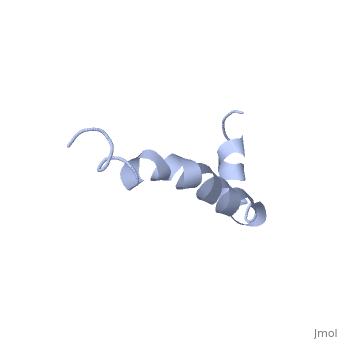
| - | The most reasonable structure determined structure consists of <scene name='Amyloid_beta/ | + | The most reasonable structure determined structure consists of <scene name='Amyloid_beta/Structure/1'>two helices</scene>; the first helix (residues 8-25) is well defined and has an RMSD of 0.38 angstroms and the second (residues 28-38) is interrupted at the Ile32-Gly33 connection. The second helix corresponds to the transmembrane region of APP and thus contains multiple small and hydrophobic amino acids. The two helices are connected by a <scene name='Amyloid_beta/Kink/1'>kink</scene> (residues 26 and 27).<ref name="structure" /> |
The toxicity of amyloid beta is due to the formation of aggregates and oligomers. The actual structures of these oligomers is unknown but is likely similar to that of amyloid beta in mature plaques.<ref name="structure" /> | The toxicity of amyloid beta is due to the formation of aggregates and oligomers. The actual structures of these oligomers is unknown but is likely similar to that of amyloid beta in mature plaques.<ref name="structure" /> | ||
Revision as of 21:31, 28 November 2011
| |||||||||||