Sandbox reserved 659
From Proteopedia
| Line 14: | Line 14: | ||
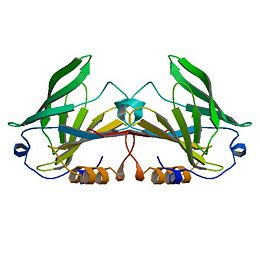
<StructureSection load='1B8E' size='350' side='right' caption='Structure of Beta Lactoglobulin (PDB entry [[1B8E]])' scene=''> | <StructureSection load='1B8E' size='350' side='right' caption='Structure of Beta Lactoglobulin (PDB entry [[1B8E]])' scene=''> | ||
| - | β-Lactoglobulin is a small protein | + | β-Lactoglobulin is a small protein with 162 amino acid residues (Mr ∼18,400). β-LG folds up into an 8-stranded, antiparallel β-barrel with a 3-turn α-helix on the outer surface and a ninth β-strand flanking the first strand. The ninth β-strand forms the dimer interface in cow and sheep proteins. The calyx, or β-barrel, is conical and serves as the ligand binding sight for small hydrophobic ligands. It is made of β-strands A-D forming one sheet, and strands E-H forming a second. Strand A turns at a right angle and the C-terminal end forms an antiparallel strand with H. A 3-turn helix between strands G and H is located on the outer surface of the β-barrel between strands G and H. The loops that connect the β-strands at the closed end of the calyx, BC, DE, and FG are generally quite short, whereas those at the open end are significantly longer and more flexible (Kontopidis, 2004). This becomes especially important when considering conformational changes at different pH values. Specifically, the EF loops can act as a “gate”, opening or closing access to and from the internal binding site. At acidic pH the “gate” closes while at neutral and basic pH values the “gate” is open, allowing for ligand binding at the hydrophobic binding site. The gate “hinge” is Glu89 (Kontopidis, 2004). |
| - | + | The free Cys121 has a reactive thiol group, whose activity parallels the pH dependency of the Tanford transition and is involved with the denaturation and aggregation of the system (Havea et al., 2001).. It is situated on the outer surfaced of the β-barrel and underneath the α-helix, which offers it some protection from the solvent and pH effects. | |
| - | The free Cys121, with its reactive thiol group, has a pH-dependent activity that parallels that of the Tanford transition, and its involvement in the denaturation and aggregation behavior is now firmly established (Havea et al., 2001). The residue is situated on the outer surface of the β-barrel on strand H, under the α-helix, and quite some distance from the EF loop. The effect of this is to mask its accessibility to solvent, particularly at low pH. Reaction with heavy metals like Hg2+ or Cd2+ does occur at most pH values and leads to a dissociation of the dimer. Indeed, reaction of the Cys with almost any thiol reagent appears to enhance dissociation into monomers (McKenzie and Sawyer, 1967; Hambling et al., 1992). | ||
| - | |||
| - | The CD and GH loops in many of the crystallographic analyses are indistinct, and the similarly indistinct nature of the extended AB loop led to the original misthreading of the amino acid sequence into the first electron density map (Papiz et al., 1986; Brownlow et al., 1997). β-Strand I is on the opposite side of strand A from strand H and forms an antiparallel interaction with the same strand of the second monomer. The other dimer-stabilizing interaction comes from the AB loop, where there is an ion pair from Asp33 of one subunit to Arg40 of the other. It is interesting to note that although strand I also exists in the porcine and equine β-LG structures, albeit with a slightly modified sequence, as do the Asp and Arg residues, no similar dimerization occurs at physiological pH. Indeed, site-directed mutagenesis designed to disrupt one or the other of these interactions, or better, both, produces a monomeric bovine protein (Sakurai and Goto, 2002; Wilson and Holt, to be published). There is no direct interaction between the α-helixes, which are antiparallel on the outer surface of the molecule with the polypeptide chains, some 12 Å apart. The closest approach of any atoms of the helix in the A subunit from the triclinic lattice X structure to the helix in the B subunit is 5.8Å, and this involves the flexible side chains of Asp137A and Arg148B and vice versa. | ||
| - | |||
| - | The structure of β-LG has also been determined by NMR techniques by several groups (Belloque and Smith, 1998; Fogolari et al., 1998; Kuwata et al., 1998; Uhrinova et al., 2000) and includes the equine structure (Kobayashi et al., 2000). The bovine protein is monomeric at pH values below 3, and the structure has been determined at this pH. Although there is no matching crystal structure at this pH where flat hexagonal plates of poor diffraction quality can be grown, the structure of the core of the protein is well conserved. However, significant differences do exist, and these have been critically reviewed (Jameson et al., 2002); it is not surprising that it is the external loops that contain these major differences. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 15:49, 27 November 2012
Beta Lactoglobulin
β-Lactoglobulin(β-LG) is the bulk constituent of whey protein in most ruminant species and is also present in the milks of most mammalian species, although not in rodent, human, and lagomorph milks. It has been studied extensively for some time ( Tilley, 1960; Lyster, 1972; Kinsella and Whitehead, 1987; Hambling et al., 1992; Sawyer, 2003). β-LG is a lipocalin as is seen through its amino-acid sequence and structure (Flower et al., 2000). Lipocalins are a varied family which bind small hydrophobic ligands, and often act as transporters in vivo. The purpose of β-LG, however, is not clear. Genetic variants of β-LG expressing genes have been reported, especially in cows and several nonruminant species (Kontopidis, 2004).
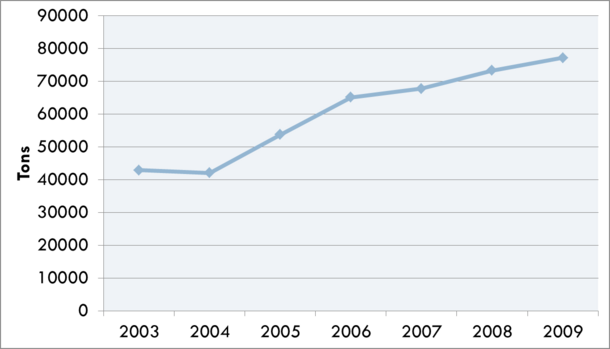
Whey is an often used food ingredient in the food industry and has many useful functional characteristics including foaming, gelling, thermal stability, emulsification, and protein fortification. It has also been suggested that it has many useful health benefits including exercise recovery, weight management, cardiovascular health, anti-cancer effects, anti-infection activity, wound repair, and infant nutrition. Human consumption of whey ingredients has steadily increased and has become more and more important to the dairy industry. An issue with whey ingredients is the subtle off flavor often associated with whey proteins. During the Cheddar cheese making process, norbixin leaches into the whey during the draining of the curd. Norbixin, a carotenoid extracted from the bixa orellana seed and added to cheese milk to give Cheddar its expected yellow flavor, would also contribute yellow color to the final product into which the whey ingredient is added. To prevent discoloration of the final product, whey is bleached by either hydrogen peroxide or benzoyl peroxide. In addition to a white color, this also causes lipid oxidation and associated off flavors.
| |||||||||||
Physiological Function of β-LG
| |||||||||||
Tanford Transition of β-LG
| |||||||||||