Acid-beta-glucosidase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='1ogs' scene='Acid-beta-glucosidase/Overview2/ | + | <StructureSection load='1ogs' scene='Acid-beta-glucosidase/Overview2/2' size='400' frame='true' align='right' |
> | > | ||
(see also [[Treatment of Gaucher disease]]) | (see also [[Treatment of Gaucher disease]]) | ||
| Line 5: | Line 5: | ||
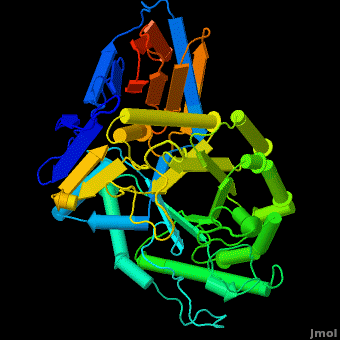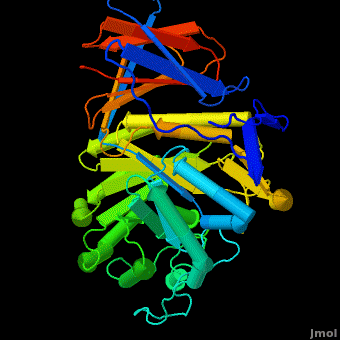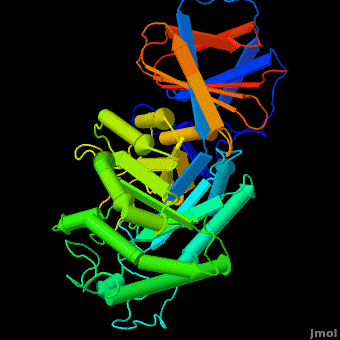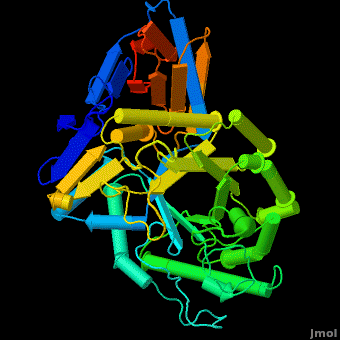
The enzyme is a lysosomal, membrane-associated glycoprotein, and its 3D structure revealed that its catalytic domain is a TIM barrel. It catalyzes hydrolysis of the sphingolipid, <scene name='Acid-beta-glucosidase/Cv/3'>glucosylceramide (GlcCer)</scene>, to <scene name='Acid-beta-glucosidase/Cv/2'>glucose and ceramide</scene> at the acidic pH prevailing within the lysosome. <scene name='Acid-beta-glucosidase/Cv/4'>Click here to see animation of this reaction</scene>. | The enzyme is a lysosomal, membrane-associated glycoprotein, and its 3D structure revealed that its catalytic domain is a TIM barrel. It catalyzes hydrolysis of the sphingolipid, <scene name='Acid-beta-glucosidase/Cv/3'>glucosylceramide (GlcCer)</scene>, to <scene name='Acid-beta-glucosidase/Cv/2'>glucose and ceramide</scene> at the acidic pH prevailing within the lysosome. <scene name='Acid-beta-glucosidase/Cv/4'>Click here to see animation of this reaction</scene>. | ||
| - | According to the mechanism, an enzyme, which acts through the [http://en.wikipedia.org/wiki/Acid-base_reaction acid-base reacion] has to contain in the <scene name='Acid-beta-glucosidase/Active_site/ | + | According to the mechanism, an enzyme, which acts through the [http://en.wikipedia.org/wiki/Acid-base_reaction acid-base reacion] has to contain in the <scene name='Acid-beta-glucosidase/Active_site/4'>active site</scene> a proton donor, an acid, and a nuclophile - the base. The nuclophile is involved in formation and stabilization of the intermediate state, were a proton is transferred from an anomeric carbon to the leaving group. At that stage, the proton donor would donate its proton to the intermediate complex, releasing the second product and hence acting as a catalytic acid. Such a mechanism depends strongly on the pH of the environment. Fine changes in acidity of the solution might affect the ionization states of the residues involved in the catalysis. Therefore there would be a strong dependence of the enzyme's activity on the pH of the solution. Some additional details in <br /> |
*[[Molecular Playground/Velaglucerase]] <br /> | *[[Molecular Playground/Velaglucerase]] <br /> | ||
*[[Disulfide Connectivity of Velaglucerase]] <br /> | *[[Disulfide Connectivity of Velaglucerase]] <br /> | ||
Revision as of 12:43, 5 December 2012
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Boris Brumshtein, Alexander Berchansky, Joel L. Sussman, Eran Hodis, David Canner