1m10
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Structural highlights == | == Structural highlights == | ||
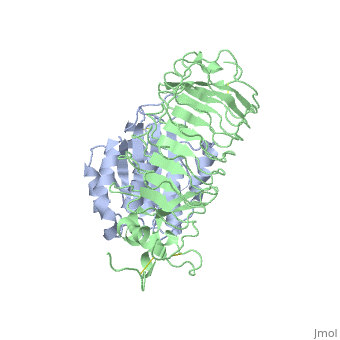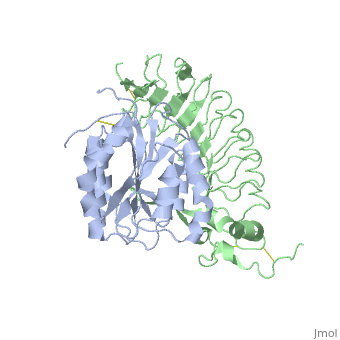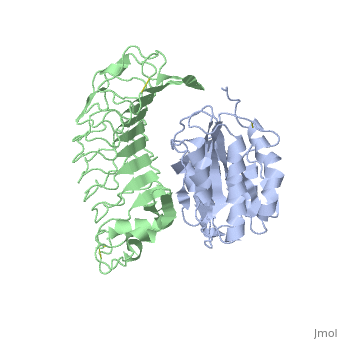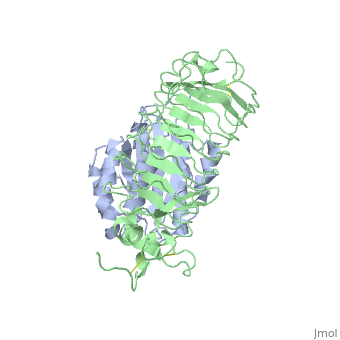
<table><tr><td colspan='2'>[[1m10]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1M10 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1M10 FirstGlance]. <br> | <table><tr><td colspan='2'>[[1m10]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1M10 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1M10 FirstGlance]. <br> | ||
| - | </td></tr><tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1m0z|1m0z]]</td></tr> | + | </td></tr><tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1m0z|1m0z]]</td></tr> |
| - | <tr><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">VWF ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens]), GP1BA ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens])</td></tr> | + | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">VWF ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens]), GP1BA ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens])</td></tr> |
| - | <tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1m10 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1m10 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1m10 RCSB], [http://www.ebi.ac.uk/pdbsum/1m10 PDBsum]</span></td></tr> | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1m10 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1m10 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1m10 RCSB], [http://www.ebi.ac.uk/pdbsum/1m10 PDBsum]</span></td></tr> |
| - | <table> | + | </table> |
== Disease == | == Disease == | ||
[[http://www.uniprot.org/uniprot/VWF_HUMAN VWF_HUMAN]] Defects in VWF are the cause of von Willebrand disease type 1 (VWD1) [MIM:[http://omim.org/entry/193400 193400]]. A common hemorrhagic disorder due to defects in von Willebrand factor protein and resulting in impaired platelet aggregation. Von Willebrand disease type 1 is characterized by partial quantitative deficiency of circulating von Willebrand factor, that is otherwise structurally and functionally normal. Clinical manifestations are mucocutaneous bleeding, such as epistaxis and menorrhagia, and prolonged bleeding after surgery or trauma.<ref>PMID:10887119</ref> <ref>PMID:11698279</ref> Defects in VWF are the cause of von Willebrand disease type 2 (VWD2) [MIM:[http://omim.org/entry/613554 613554]]. A hemorrhagic disorder due to defects in von Willebrand factor protein and resulting in impaired platelet aggregation. Von Willebrand disease type 2 is characterized by qualitative deficiency and functional anomalies of von Willebrand factor. It is divided in different subtypes including 2A, 2B, 2M and 2N (Normandy variant). The mutant VWF protein in types 2A, 2B and 2M are defective in their platelet-dependent function, whereas the mutant protein in type 2N is defective in its ability to bind factor VIII. Clinical manifestations are mucocutaneous bleeding, such as epistaxis and menorrhagia, and prolonged bleeding after surgery or trauma. Defects in VWF are the cause of von Willebrand disease type 3 (VWD3) [MIM:[http://omim.org/entry/277480 277480]]. A severe hemorrhagic disorder due to a total or near total absence of von Willebrand factor in the plasma and cellular compartments, also leading to a profound deficiency of plasmatic factor VIII. Bleeding usually starts in infancy and can include epistaxis, recurrent mucocutaneous bleeding, excessive bleeding after minor trauma, and hemarthroses. [[http://www.uniprot.org/uniprot/GP1BA_HUMAN GP1BA_HUMAN]] Genetic variations in GP1BA may be a cause of susceptibility to non-arteritic anterior ischemic optic neuropathy (NAION) [MIM:[http://omim.org/entry/258660 258660]]. NAION is an ocular disease due to ischemic injury to the optic nerve. It usually affects the optic disk and leads to visual loss and optic disk swelling of a pallid nature. Visual loss is usually sudden, or over a few days at most and is usually permanent, with some recovery possibly occurring within the first weeks or months. Patients with small disks having smaller or non-existent cups have an anatomical predisposition for non-arteritic anterior ischemic optic neuropathy. As an ischemic episode evolves, the swelling compromises circulation, with a spiral of ischemia resulting in further neuronal damage.<ref>PMID:14711733</ref> Defects in GP1BA are a cause of Bernard-Soulier syndrome (BSS) [MIM:[http://omim.org/entry/231200 231200]]; also known as giant platelet disease (GPD). BSS patients have unusually large platelets and have a clinical bleeding tendency.<ref>PMID:1730088</ref> <ref>PMID:7690774</ref> <ref>PMID:7819107</ref> <ref>PMID:7873390</ref> <ref>PMID:9639514</ref> <ref>PMID:10089893</ref> Defects in GP1BA are the cause of benign mediterranean macrothrombocytopenia (BMM) [MIM:[http://omim.org/entry/153670 153670]]; also known as autosomal dominant benign Bernard-Soulier syndrome. BMM is characterized by mild or no clinical symptoms, normal platelet function, and normal megakaryocyte count.<ref>PMID:11222377</ref> Defects in GP1BA are the cause of pseudo-von Willebrand disease (VWDP) [MIM:[http://omim.org/entry/177820 177820]]. A bleeding disorder is caused by an increased affinity of GP-Ib for soluble vWF resulting in impaired hemostatic function due to the removal of vWF from the circulation.<ref>PMID:14521605</ref> <ref>PMID:2052556</ref> <ref>PMID:8486780</ref> <ref>PMID:8384898</ref> | [[http://www.uniprot.org/uniprot/VWF_HUMAN VWF_HUMAN]] Defects in VWF are the cause of von Willebrand disease type 1 (VWD1) [MIM:[http://omim.org/entry/193400 193400]]. A common hemorrhagic disorder due to defects in von Willebrand factor protein and resulting in impaired platelet aggregation. Von Willebrand disease type 1 is characterized by partial quantitative deficiency of circulating von Willebrand factor, that is otherwise structurally and functionally normal. Clinical manifestations are mucocutaneous bleeding, such as epistaxis and menorrhagia, and prolonged bleeding after surgery or trauma.<ref>PMID:10887119</ref> <ref>PMID:11698279</ref> Defects in VWF are the cause of von Willebrand disease type 2 (VWD2) [MIM:[http://omim.org/entry/613554 613554]]. A hemorrhagic disorder due to defects in von Willebrand factor protein and resulting in impaired platelet aggregation. Von Willebrand disease type 2 is characterized by qualitative deficiency and functional anomalies of von Willebrand factor. It is divided in different subtypes including 2A, 2B, 2M and 2N (Normandy variant). The mutant VWF protein in types 2A, 2B and 2M are defective in their platelet-dependent function, whereas the mutant protein in type 2N is defective in its ability to bind factor VIII. Clinical manifestations are mucocutaneous bleeding, such as epistaxis and menorrhagia, and prolonged bleeding after surgery or trauma. Defects in VWF are the cause of von Willebrand disease type 3 (VWD3) [MIM:[http://omim.org/entry/277480 277480]]. A severe hemorrhagic disorder due to a total or near total absence of von Willebrand factor in the plasma and cellular compartments, also leading to a profound deficiency of plasmatic factor VIII. Bleeding usually starts in infancy and can include epistaxis, recurrent mucocutaneous bleeding, excessive bleeding after minor trauma, and hemarthroses. [[http://www.uniprot.org/uniprot/GP1BA_HUMAN GP1BA_HUMAN]] Genetic variations in GP1BA may be a cause of susceptibility to non-arteritic anterior ischemic optic neuropathy (NAION) [MIM:[http://omim.org/entry/258660 258660]]. NAION is an ocular disease due to ischemic injury to the optic nerve. It usually affects the optic disk and leads to visual loss and optic disk swelling of a pallid nature. Visual loss is usually sudden, or over a few days at most and is usually permanent, with some recovery possibly occurring within the first weeks or months. Patients with small disks having smaller or non-existent cups have an anatomical predisposition for non-arteritic anterior ischemic optic neuropathy. As an ischemic episode evolves, the swelling compromises circulation, with a spiral of ischemia resulting in further neuronal damage.<ref>PMID:14711733</ref> Defects in GP1BA are a cause of Bernard-Soulier syndrome (BSS) [MIM:[http://omim.org/entry/231200 231200]]; also known as giant platelet disease (GPD). BSS patients have unusually large platelets and have a clinical bleeding tendency.<ref>PMID:1730088</ref> <ref>PMID:7690774</ref> <ref>PMID:7819107</ref> <ref>PMID:7873390</ref> <ref>PMID:9639514</ref> <ref>PMID:10089893</ref> Defects in GP1BA are the cause of benign mediterranean macrothrombocytopenia (BMM) [MIM:[http://omim.org/entry/153670 153670]]; also known as autosomal dominant benign Bernard-Soulier syndrome. BMM is characterized by mild or no clinical symptoms, normal platelet function, and normal megakaryocyte count.<ref>PMID:11222377</ref> Defects in GP1BA are the cause of pseudo-von Willebrand disease (VWDP) [MIM:[http://omim.org/entry/177820 177820]]. A bleeding disorder is caused by an increased affinity of GP-Ib for soluble vWF resulting in impaired hemostatic function due to the removal of vWF from the circulation.<ref>PMID:14521605</ref> <ref>PMID:2052556</ref> <ref>PMID:8486780</ref> <ref>PMID:8384898</ref> | ||
| Line 39: | Line 39: | ||
</StructureSection> | </StructureSection> | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
| - | [[Category: Groot, P G.de | + | [[Category: Groot, P G.de]] |
| - | [[Category: Gros, P | + | [[Category: Gros, P]] |
| - | [[Category: Huizinga, E G | + | [[Category: Huizinga, E G]] |
| - | [[Category: Romijn, R A.P | + | [[Category: Romijn, R A.P]] |
| - | [[Category: Schiphorst, M E | + | [[Category: Schiphorst, M E]] |
| - | [[Category: Sixma, J J | + | [[Category: Sixma, J J]] |
| - | [[Category: Tsuji, S | + | [[Category: Tsuji, S]] |
[[Category: Blood clotting]] | [[Category: Blood clotting]] | ||
[[Category: Dinucleotide binding fold]] | [[Category: Dinucleotide binding fold]] | ||
[[Category: Hemostasis]] | [[Category: Hemostasis]] | ||
[[Category: Leucine-rich repeat]] | [[Category: Leucine-rich repeat]] | ||
Revision as of 15:44, 5 January 2015
Crystal structure of the complex of Glycoprotein Ib alpha and the von Willebrand Factor A1 Domain
| |||||||||||