Acid-beta-glucosidase
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
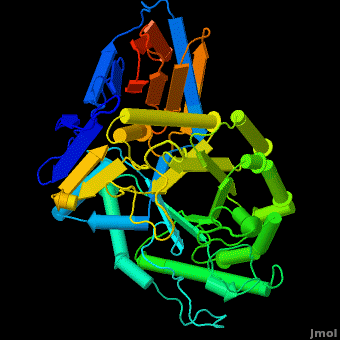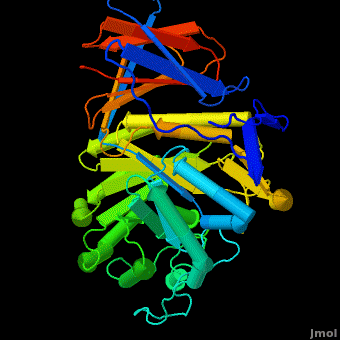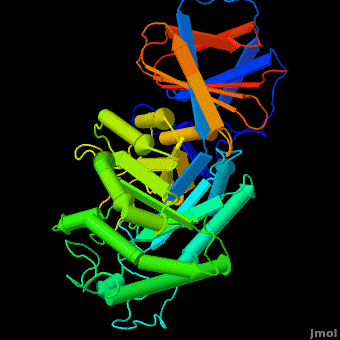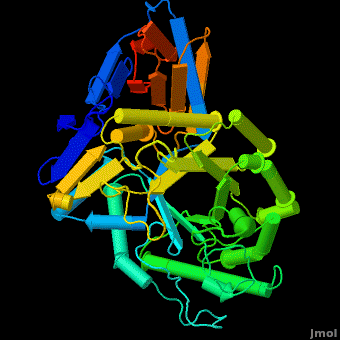
The entrance to the active site of the enzyme is confined by three loops (Loop-1, residues 346-349; Loop-2, 393-399; Loop-3, 312-319), which have been observed in a number of conformations. Loop-1 is found to be involved in crystal contact interactions between the two individual protein molecules in crystals. Its conformation was not found to change significantly in various crystals of the enzyme. In contrast, loops-2 and 3 were detected in several different stable conformations, and displayed varying conformations in different structures even in the absence of an inhibitor in the active site. These loops were found to change the shape of the entrance as well as several properties of the active site. | The entrance to the active site of the enzyme is confined by three loops (Loop-1, residues 346-349; Loop-2, 393-399; Loop-3, 312-319), which have been observed in a number of conformations. Loop-1 is found to be involved in crystal contact interactions between the two individual protein molecules in crystals. Its conformation was not found to change significantly in various crystals of the enzyme. In contrast, loops-2 and 3 were detected in several different stable conformations, and displayed varying conformations in different structures even in the absence of an inhibitor in the active site. These loops were found to change the shape of the entrance as well as several properties of the active site. | ||
| - | |||
== Gaucher disease == | == Gaucher disease == | ||
| - | |||
[http://en.wikipedia.org/wiki/Gaucher's_disease Gaucher disease]is the most common lysosomal storage disease, and is associated with mutations in the gene coding for the enzyme acid-β-glucosidase ([http://www.chem.qmul.ac.uk/iubmb/enzyme/ enzyme classification] E.C. 3.2.1.45). | [http://en.wikipedia.org/wiki/Gaucher's_disease Gaucher disease]is the most common lysosomal storage disease, and is associated with mutations in the gene coding for the enzyme acid-β-glucosidase ([http://www.chem.qmul.ac.uk/iubmb/enzyme/ enzyme classification] E.C. 3.2.1.45). | ||
There are ~200 known mutations, mostly missense mutations which result in substitution of amino acids in the protein. Some mutations cause complete deactivation of the enzyme; others impair its stability, and some affect both activity and stability. There are three known phenotypes of the disease: a mild, severe and acute. The acute phenotype is neuropathic, while severe and mild symptoms are caused by accumulation of GlcCer in macrophage cells resulting in bone atrophy, spleen enlargement, etc. | There are ~200 known mutations, mostly missense mutations which result in substitution of amino acids in the protein. Some mutations cause complete deactivation of the enzyme; others impair its stability, and some affect both activity and stability. There are three known phenotypes of the disease: a mild, severe and acute. The acute phenotype is neuropathic, while severe and mild symptoms are caused by accumulation of GlcCer in macrophage cells resulting in bone atrophy, spleen enlargement, etc. | ||
| Line 38: | Line 36: | ||
There are two available treatments for the disease: an enzyme replacement therapy and a substrate reduction therapy. | There are two available treatments for the disease: an enzyme replacement therapy and a substrate reduction therapy. | ||
In enzyme replacement therapy a recombinant enzyme (Cerezyme™) is produced by the Genzyme company and intra-venously injected to patients. In a substrate reduction therapy a small molecule inhibitor (Zavesca™) is used to inhibit the synthesis of the accumulated glucosylceramide. | In enzyme replacement therapy a recombinant enzyme (Cerezyme™) is produced by the Genzyme company and intra-venously injected to patients. In a substrate reduction therapy a small molecule inhibitor (Zavesca™) is used to inhibit the synthesis of the accumulated glucosylceramide. | ||
| - | |||
== Cerezyme™ vs Plant produced enzyme in a Gaucher disease == | == Cerezyme™ vs Plant produced enzyme in a Gaucher disease == | ||
| - | |||
Acid-beta-glucosidase for treatment of a Gaucher disease is produced in CHO cells (<scene name='Acid-beta-glucosidase/Overlay/6'>Cerezyme</scene>™) ([[1ogs]];[[1y7v]];[[2f61]]; [[2nt0]]; [[2nt1]]; [[2v3d]]; [[2v3e]]; [[2nsx]]; [[2j25]]) or in a carrot stem cells suspension (plant produced enzyme by Protalix biopharmaceuticals, [[2v3d]]; [[2v3f]]; [[2v3e]]). Recently solved structures of both enzymes indicate the enzymes are virtually <scene name='Acid-beta-glucosidase/Overlay/7'>identical</scene> in their 3D structures. Yet some small insignificant differences in sugars arise due to the use of different expression systems. | Acid-beta-glucosidase for treatment of a Gaucher disease is produced in CHO cells (<scene name='Acid-beta-glucosidase/Overlay/6'>Cerezyme</scene>™) ([[1ogs]];[[1y7v]];[[2f61]]; [[2nt0]]; [[2nt1]]; [[2v3d]]; [[2v3e]]; [[2nsx]]; [[2j25]]) or in a carrot stem cells suspension (plant produced enzyme by Protalix biopharmaceuticals, [[2v3d]]; [[2v3f]]; [[2v3e]]). Recently solved structures of both enzymes indicate the enzymes are virtually <scene name='Acid-beta-glucosidase/Overlay/7'>identical</scene> in their 3D structures. Yet some small insignificant differences in sugars arise due to the use of different expression systems. | ||
| - | + | </StructureSection> | |
| - | + | ||
== 3D structures of Acid-β-glucosidase == | == 3D structures of Acid-β-glucosidase == | ||
Revision as of 11:40, 30 November 2015
| |||||||||||
3D structures of Acid-β-glucosidase
Updated on 30-November-2015
2wkl, 3gxd, 3gxi, 3gxm, 2v3f, 2nt0, 2nt1, 2j25, 2f61, 1ogs, 3rik – hABG – human
3ke0, 3keh – hABG (mutant)
2wcg, 3gxf, 2vt0, 2v3e, 2v3d, 1y7v, 3ril – hABG + inhibitor
2nsx – hABG + pharmacological chaperone
2xwd, 2xwe – hABG + nojirimycin derivative
Additional Resources
Metabolic Disorders
Carbohydrate Metabolism
Treatment of Gaucher disease
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Boris Brumshtein, Alexander Berchansky, Joel L. Sussman, Eran Hodis, David Canner