Acetylcholinesterase
From Proteopedia
(Difference between revisions)
| Line 52: | Line 52: | ||
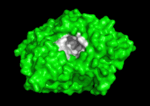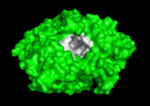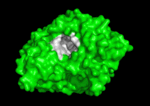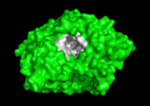
<scene name='Main_Page/E2020_in_ache_spinning/1'>Aricept (E2020)</scene> ([[Donepezil]]) is one of the most interesting drugs that have been designed as AChE bivalent inhibitors. It was developed, synthesized and evaluated by the Eisai Company in Japan. These inhibitors were designed on the basis of QSAR studies prior to elucidation of the 3D structure of ''Torpedo californica'' AChE (''Tc''AChE) ([[1ea5]]). It significantly enhances performance in animal models of cholinergic hypofunction and has a high affinity for AChE, binding to both electric eel and mouse AChE in the nanomolar range. The X-ray structure of the E2020-''Tc''AChE complex ([[1eve]]) shows that E2020 has a <scene name='1eve/E2020_close_up_with_84_279/13'>unique orientation</scene> along the active-site gorge, extending from the anionic subsite (<scene name='1eve/E2020_close_up_with_84lbld/7'>W84</scene>) of the active site, at the bottom, to the peripheral anionic site (<scene name='1eve/E2020_close_up_with_84_279lbld/5'>near W279</scene>), at the top, via aromatic stacking interactions with conserved aromatic acid residues. E2020 does not, however, interact directly with either the catalytic triad or the 'oxyanion hole' but only <scene name='1eve/E20_interactionshown/8'>indirectly via solvent molecules</scene>. The X-ray structure shows, a posteriori, that the design of E2020 took advantage of several important features of the active-site gorge of AChE, to produce a drug with both high affinity for AChE and a high degree of selectivity for AChE versus butyrylcholinesterase (BChE). It also delineates voids within the gorge that are not occupied by E2020 and could provide sites for potential modification of E2020 to produce drugs with improved pharmacological profiles <ref name="Kryger">PMID:10368299</ref>.<br /> | <scene name='Main_Page/E2020_in_ache_spinning/1'>Aricept (E2020)</scene> ([[Donepezil]]) is one of the most interesting drugs that have been designed as AChE bivalent inhibitors. It was developed, synthesized and evaluated by the Eisai Company in Japan. These inhibitors were designed on the basis of QSAR studies prior to elucidation of the 3D structure of ''Torpedo californica'' AChE (''Tc''AChE) ([[1ea5]]). It significantly enhances performance in animal models of cholinergic hypofunction and has a high affinity for AChE, binding to both electric eel and mouse AChE in the nanomolar range. The X-ray structure of the E2020-''Tc''AChE complex ([[1eve]]) shows that E2020 has a <scene name='1eve/E2020_close_up_with_84_279/13'>unique orientation</scene> along the active-site gorge, extending from the anionic subsite (<scene name='1eve/E2020_close_up_with_84lbld/7'>W84</scene>) of the active site, at the bottom, to the peripheral anionic site (<scene name='1eve/E2020_close_up_with_84_279lbld/5'>near W279</scene>), at the top, via aromatic stacking interactions with conserved aromatic acid residues. E2020 does not, however, interact directly with either the catalytic triad or the 'oxyanion hole' but only <scene name='1eve/E20_interactionshown/8'>indirectly via solvent molecules</scene>. The X-ray structure shows, a posteriori, that the design of E2020 took advantage of several important features of the active-site gorge of AChE, to produce a drug with both high affinity for AChE and a high degree of selectivity for AChE versus butyrylcholinesterase (BChE). It also delineates voids within the gorge that are not occupied by E2020 and could provide sites for potential modification of E2020 to produce drugs with improved pharmacological profiles <ref name="Kryger">PMID:10368299</ref>.<br /> | ||
| + | |||
| + | See [[Treatments:AChE Inhibitor References]]. | ||
| + | |||
=====See details of AChE-Aricept complex in various languages===== | =====See details of AChE-Aricept complex in various languages===== | ||
Revision as of 11:52, 25 February 2016
| |||||||||||
Contents |
3D Structures of AChE
See 3D structures of acetylcholinesterase
Additional Resources
For additional information, see:
Alzheimer's Disease
AChE inhibitors and substrates
External Links
- Acetylcholinesterase Tutorial by Karl Oberholser, Messiah College
- PDB Molecule of the Month - Acetylcholinesterase
- Movies: X-ray Damage in ACh & Nature's Vacuum Cleaner by R. Gillilan, Cornell Univ
References
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Botti SA, Felder CE, Lifson S, Sussman JL, Silman I. A modular treatment of molecular traffic through the active site of cholinesterase. Biophys J. 1999 Nov;77(5):2430-50. PMID:10545346
- ↑ 3.0 3.1 Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol. 1997 Jan;4(1):57-63. PMID:8989325
- ↑ Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 A resolution. FEBS Lett. 1999 Dec 17;463(3):321-6. PMID:10606746
- ↑ Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9031-5. PMID:8415649
- ↑ Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002 Mar 19;41(11):3555-64. PMID:11888271
- ↑ Haviv H, Wong DM, Greenblatt HM, Carlier PR, Pang YP, Silman I, Sussman JL. Crystal packing mediates enantioselective ligand recognition at the peripheral site of acetylcholinesterase. J Am Chem Soc. 2005 Aug 10;127(31):11029-36. PMID:16076210 doi:http://dx.doi.org/10.1021/ja051765f
- ↑ 8.0 8.1 Ravelli RB, Raves ML, Ren Z, Bourgeois D, Roth M, Kroon J, Silman I, Sussman JL. Static Laue diffraction studies on acetylcholinesterase. Acta Crystallogr D Biol Crystallogr. 1998 Nov 1;54(Pt 6 Pt 2):1359-66. PMID:10089512
- ↑ 9.0 9.1 Harel M, Sonoda LK, Silman I, Sussman JL, Rosenberry TL. Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J Am Chem Soc. 2008 Jun 25;130(25):7856-61. Epub 2008 May 31. PMID:18512913 doi:http://dx.doi.org/10.1021/ja7109822
- ↑ Greenblatt HM, Guillou C, Guenard D, Argaman A, Botti S, Badet B, Thal C, Silman I, Sussman JL. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: implications for structure-based drug design. J Am Chem Soc. 2004 Dec 1;126(47):15405-11. PMID:15563167 doi:http://dx.doi.org/10.1021/ja0466154
- ↑ Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure. 1999 Mar 15;7(3):297-307. PMID:10368299
- ↑ Sanson B, Nachon F, Colletier JP, Froment MT, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime (dagger). J Med Chem. 2009 Jul 30. PMID:19642642 doi:10.1021/jm900433t
- ↑ Paz A, Roth E, Ashani Y, Xu Y, Shnyrov VL, Sussman JL, Silman I, Weiner L. Structural and functional characterization of the interaction of the photosensitizing probe methylene blue with Torpedo californica acetylcholinesterase. Protein Sci. 2012 Jun 1. doi: 10.1002/pro.2101. PMID:22674800 doi:10.1002/pro.2101
Treatments:AChE Inhibitor References
Treatments:Alzheimer's Disease
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, David Canner, Eran Hodis, Clifford Felder, Jaime Prilusky, Harry Greenblatt, Yechun Xu