SUMO
From Proteopedia
| Line 1: | Line 1: | ||
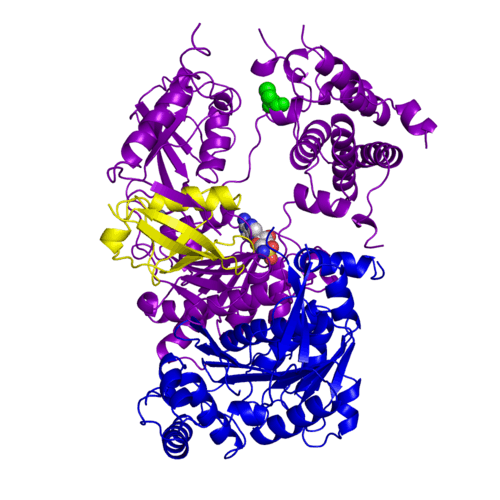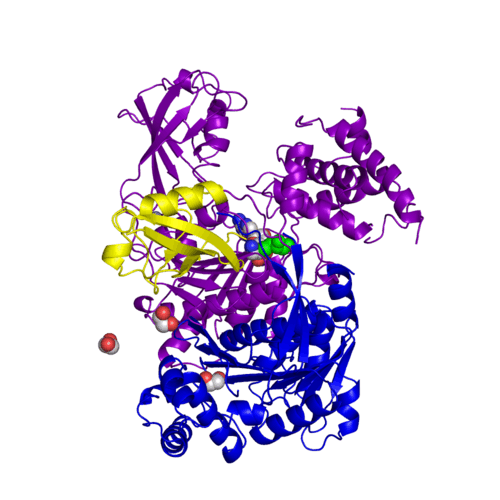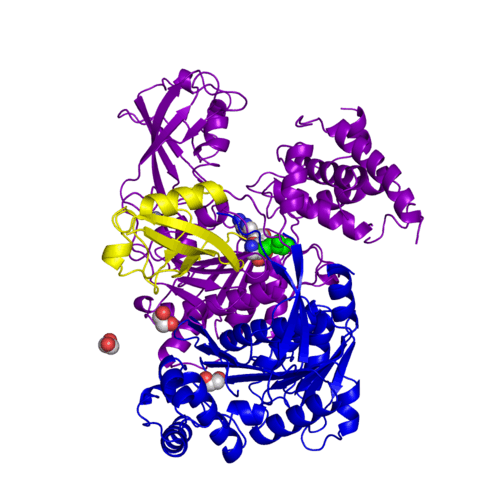
<StructureSection load='3kyc' size='350' scene='' caption='Human SUMO-1 (yellow) complex with SUMO-activating enzyme subunit 1 (grey), SUMO-activating enzyme subunit 2 (green), adenosine derivative and Zn+2 ion (grey) (PDB code [[3kyc]])'> | <StructureSection load='3kyc' size='350' scene='' caption='Human SUMO-1 (yellow) complex with SUMO-activating enzyme subunit 1 (grey), SUMO-activating enzyme subunit 2 (green), adenosine derivative and Zn+2 ion (grey) (PDB code [[3kyc]])'> | ||
| + | __TOC__ | ||
== Function == | == Function == | ||
[[SUMO]] is a '''Small Ubiquitin-like MOdifier''' which covalently attaches to cellular proteins to modify their function. SUMO is similar in structure but not in sequence to [[Ubiquitin|ubiquitin]]. In several organisms SUMO is called SMT3. The SUMO-conjugating enzyme (E2) is called UBC9. The sentrin specific protease (SEPN) cleaves the C-terminal peptide from SUMO which then can bind to ubiquitin activating enzyme (E1). For details on SUMO-1 protein complex see <br /> | [[SUMO]] is a '''Small Ubiquitin-like MOdifier''' which covalently attaches to cellular proteins to modify their function. SUMO is similar in structure but not in sequence to [[Ubiquitin|ubiquitin]]. In several organisms SUMO is called SMT3. The SUMO-conjugating enzyme (E2) is called UBC9. The sentrin specific protease (SEPN) cleaves the C-terminal peptide from SUMO which then can bind to ubiquitin activating enzyme (E1). For details on SUMO-1 protein complex see <br /> | ||
| Line 9: | Line 10: | ||
Sumoylation may have a potential role in Alzheimer disease and decrease sumoylation of lamina A is a causative factor in familial dilated cardiomyopathy<ref>PMID:19282183</ref>. | Sumoylation may have a potential role in Alzheimer disease and decrease sumoylation of lamina A is a causative factor in familial dilated cardiomyopathy<ref>PMID:19282183</ref>. | ||
| + | == Structural highlights == | ||
[[Ubiquitin]] (Ub) and ubiquitin-like (Ubl) proteins attached to their target proteins and modulating the activities of those targets in various ways. Three types of evolutionarily conserved enzymes — E1 activating enzymes, E2 conjugating enzymes and E3 ligase enzymes — act sequentially through parallel yet distinct pathways to conjugate ubiquitin and Ubl proteins, such as SUMO and NEDD8, to their targets. The E1 enzyme uses the <scene name='3kyc/Cv/3'>adenosine triphosphate (ATP)</scene> and magnesium to adenylate the C-terminal Ub/Ubl glycine, releasing pyrophosphate and resulting in <scene name='3kyc/Cv/8'>adenosine monophosphate (AMP)</scene>. A non-hydrolysable <scene name='3kyc/Cv/4'>mimic of the acyl adenylate intermediate (AMSN)</scene> and <scene name='3kyc/Cv/5'>mimic of the tetrahedral intermediate (AVSN)</scene> were constructed. In both these compounds the atom of <font color='orange'><b>phosphorus</b></font> is replaced by sulfur (colored <font color='yellow'><b>yellow</b></font>). | [[Ubiquitin]] (Ub) and ubiquitin-like (Ubl) proteins attached to their target proteins and modulating the activities of those targets in various ways. Three types of evolutionarily conserved enzymes — E1 activating enzymes, E2 conjugating enzymes and E3 ligase enzymes — act sequentially through parallel yet distinct pathways to conjugate ubiquitin and Ubl proteins, such as SUMO and NEDD8, to their targets. The E1 enzyme uses the <scene name='3kyc/Cv/3'>adenosine triphosphate (ATP)</scene> and magnesium to adenylate the C-terminal Ub/Ubl glycine, releasing pyrophosphate and resulting in <scene name='3kyc/Cv/8'>adenosine monophosphate (AMP)</scene>. A non-hydrolysable <scene name='3kyc/Cv/4'>mimic of the acyl adenylate intermediate (AMSN)</scene> and <scene name='3kyc/Cv/5'>mimic of the tetrahedral intermediate (AVSN)</scene> were constructed. In both these compounds the atom of <font color='orange'><b>phosphorus</b></font> is replaced by sulfur (colored <font color='yellow'><b>yellow</b></font>). | ||
Revision as of 08:43, 5 September 2016
| |||||||||||
For better understanding of the difference between these two conformations you can see this morph (generated by using POLYVIEW-3D: http://polyview.cchmc.org/polyview3d.html; reload/refresh this page to restart this movie). Of note, in contrast to the previous figure, the same domains of these two structures (3kyc and 3kyd) are colored in the same colors (SUMO1 in yellow, SAE1 colored in blue and other domains in darkviolet). The catalytic Cys173 is shown in the spacefill representation and colored green, AMSN (or AVSN) are shown in the spacefill representation and colored in CPK colors.
3D Structures of SUMO
Updated on 05-September-2016
Reference
- Olsen SK, Capili AD, Lu X, Tan DS, Lima CD. Active site remodelling accompanies thioester bond formation in the SUMO E1. Nature. 2010 Feb 18;463(7283):906-12. PMID:20164921 doi:10.1038/nature08765