SUMO
From Proteopedia
| Line 14: | Line 14: | ||
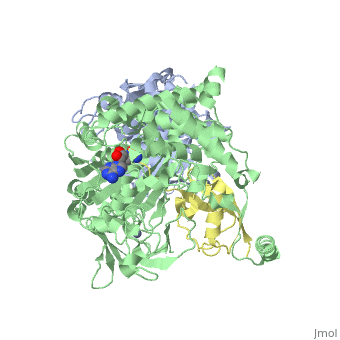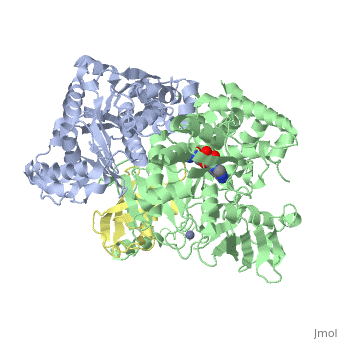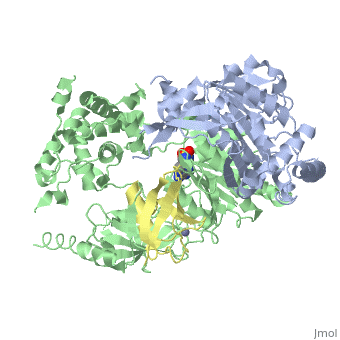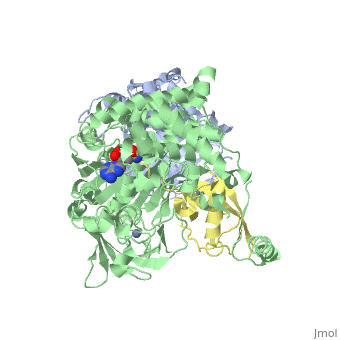
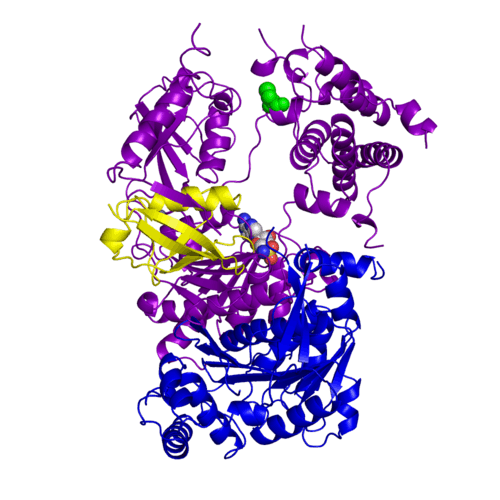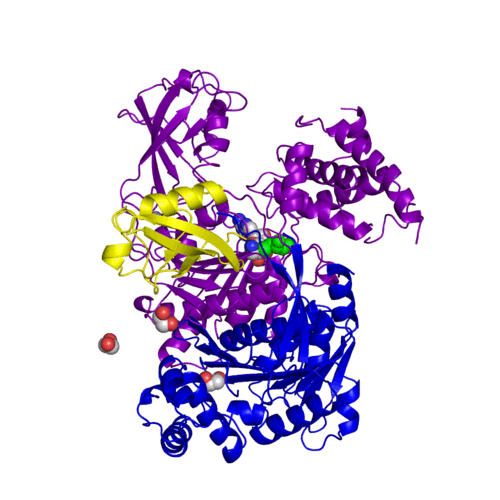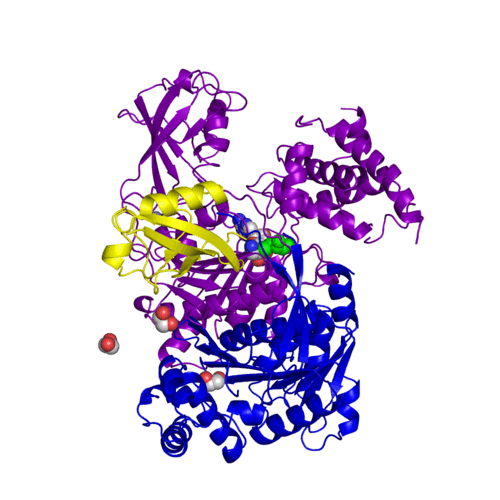
| - | The <scene name='3kyc/Al/2'>structural alignment</scene> of the crystal structures for human SUMO E1 in complex with SUMO adenylate (AMSN) and tetrahedral intermediate (AVSN) analogues revealed opened conformation (<font color='orange'><b>SUMO1 in orange</b></font>, <font color='blue'><b>SAE1 colored in blue</b></font>, and <font color='darkviolet'><b>other domains in darkviolet</b></font>) and closed conformation (<font color='yellow'><b>SUMO1 in yellow</b></font>, <font color='cyan'><b>SAE1 colored in cyan</b></font>, and <font color='magenta'><b>other domains in magenta</b></font>), respectively. In the <scene name='3kyc/Al/7'>open conformation</scene> ([[3kyc]]) the distance between Cys domain (including Cys173) and mimic of the acyl adenylate intermediate AMSN is very long, while in the <scene name='3kyc/Al/6'>closed conformation</scene> ([[3kyd]]), the catalytic Cys173 is posioned near AVSN and SUMO1, so the overall structure revealed dramatic rearrangement. This large conformational change forms the <scene name='3kyc/Al/8'>E1~SUMO1-AVSN tetrahedral intermediate analogue</scene>. | + | The <scene name='3kyc/Al/2'>structural alignment</scene> of the crystal structures for human SUMO E1 in complex with SUMO adenylate (AMSN) and tetrahedral intermediate (AVSN) analogues revealed opened conformation (<font color='orange'><b>SUMO1 in orange</b></font>, <font color='blue'><b>SAE1 colored in blue</b></font>, and <font color='darkviolet'><b>other domains in darkviolet</b></font>) and closed conformation (<font color='yellow'><b>SUMO1 in yellow</b></font>, <font color='cyan'><b>SAE1 colored in cyan</b></font>, and <font color='magenta'><b>other domains in magenta</b></font>), respectively. In the <scene name='3kyc/Al/7'>open conformation</scene> ([[3kyc]]) the distance between Cys domain (including Cys173) and mimic of the acyl adenylate intermediate AMSN is very long, while in the <scene name='3kyc/Al/6'>closed conformation</scene> ([[3kyd]]), the catalytic Cys173 is posioned near AVSN and SUMO1, so the overall structure revealed dramatic rearrangement. This large conformational change forms the <scene name='3kyc/Al/8'>E1~SUMO1-AVSN tetrahedral intermediate analogue</scene>.<ref>PMID:20164921</ref> |
</StructureSection> | </StructureSection> | ||
{{Clear}} | {{Clear}} | ||
| Line 88: | Line 88: | ||
}} | }} | ||
==Reference== | ==Reference== | ||
| - | + | <references/> | |
| + | |||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Revision as of 10:25, 5 September 2016
| |||||||||||
For better understanding of the difference between these two conformations you can see this morph (generated by using POLYVIEW-3D: http://polyview.cchmc.org/polyview3d.html; reload/refresh this page to restart this movie). Of note, in contrast to the previous figure, the same domains of these two structures (3kyc and 3kyd) are colored in the same colors (SUMO1 in yellow, SAE1 colored in blue and other domains in darkviolet). The catalytic Cys173 is shown in the spacefill representation and colored green, AMSN (or AVSN) are shown in the spacefill representation and colored in CPK colors.
3D Structures of SUMO
Updated on 05-September-2016
Reference
- ↑ Sarge KD, Park-Sarge OK. Sumoylation and human disease pathogenesis. Trends Biochem Sci. 2009 Apr;34(4):200-5. doi: 10.1016/j.tibs.2009.01.004. Epub, 2009 Mar 11. PMID:19282183 doi:http://dx.doi.org/10.1016/j.tibs.2009.01.004
- ↑ Olsen SK, Capili AD, Lu X, Tan DS, Lima CD. Active site remodelling accompanies thioester bond formation in the SUMO E1. Nature. 2010 Feb 18;463(7283):906-12. PMID:20164921 doi:10.1038/nature08765