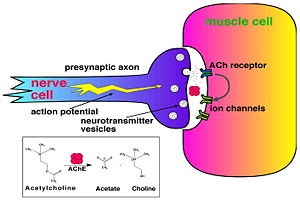
Acetylcholinesterase (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, acetylcholine (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors.
Key Enzyme in the Nervous System
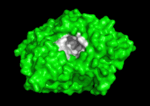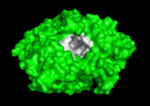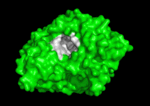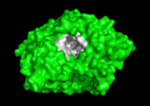
Solution of the three-dimensional (3D) structure of Torpedo californica acetylcholinesterase (TcAChE) in 1991 opened up new horizons in research on an enzyme that had already been the subject of intensive investigation.[1] The unanticipated structure of this extremely rapid enzyme, in which the active site was found to be buried at the bottom of a , lined by (colored dark magenta), led to a revision of the views then held concerning substrate traffic, recognition and hydrolysis.[2] To understand how those aromatic residues behave with the enzyme, see Flexibility of aromatic residues in acetylcholinesterase. Solution of the 3D structure of acetylcholinesterase led to a series of theoretical and experimental studies, which took advantage of recent advances in theoretical techniques for treatment of proteins, such as
molecular dynamics and electrostatics and to site-directed mutagenesis, utilizing suitable expression
systems. Acetylcholinesterase hydrolysizes the neurotransmitter acetylcholine , producing group. ACh directly binds (via its nucleophilic Oγ atom) within the (ACh/TcAChE structure 2ace). The residues are also important in the ligand recognition [3]. After this binding acetylcholinesterase ACh. See also:
AChE inhibitors and substrates
Cholinesterase
Acetylcholinesterase (Hebrew)
Treatment of Alzheimer's disease
Alzheimer's disease (AD) is a disorder that attacks the central nervous system through progressive degeneration of its neurons. AD occurs in around 10% of the elderly and, as yet, there is no known cure. Patients with this disease develop dementia which becomes more severe as the disease progresses. It was suggested that symptoms of AD are caused by decrease of activity of cholinergic neocortical and hippocampal neurons. Treatment of AD by ACh precursors and cholinergic agonists was ineffective or caused severe side effects. ACh hydrolysis by AChE causes termination of cholinergic neurotransmission. Therefore, compounds which inhibit AChE might significantly increase the levels of ACh depleted in AD. Indeed, it was shown that AChE inhibitors improve the cognitive abilities of AD patients at early stages of the disease development.
The first generation of AD drugs - monovalent AChE inhibitors
The first generation of AD drugs were AChE inhibitors: alcaloids like (-)-Huperzine A (HupA) and (-)-galanthamine (GAL, Reminyl); synthetic compounds tacrine (Cognex) and rivastigmine (Exelon). See also AChE bivalent inhibitors
HupA
HupA, discovered by Chinese scientists from 1980s, has been proved to be a powerful, highly specific, and reversible inhibitor of AChE. The crystal structure of the complex of TcAChE with HupA at 2.5 Å resolution (1vot) was determined in 1997 and it shows an unexpected orientation for the inhibitor with surprisingly few strong direct interactions with protein residues to explain its high affinity. HupA binds to TcAChE at the active site, and its in comparison to ACh. The principal interactions of are including: a direct (colored orange) through a water molecule as a linker at the bottom of the gorge; cation-π interactions between the amino group of (colored green) with the distance between the nitrogen and the centroid of the aromatic rings of 4.8 and 4.7 Å, respectively; at the top of the gorge, hydrogen bonds through two water molecules as linkers formed between the amino group of (colored magenta). An unusually short (~3.0 Å) C-H→O HB has been seen between the ethylidene methyl group of (colored crimson) [3].
Galanthamine
. (red) is an alkaloid from the flower snowdrop (Galanthus nivalis). The X-ray crystal structure of the TcAChE/GAL complex (1dx6) was determined at 2.3 Å resolution. The inhibitor binds at the base of the active site gorge of TcAChE, interacting with both the choline-binding site (Trp84) and the acyl-binding pocket (Phe288, Phe290). The tertiary amine appears to make a non-conventional hydrogen bond, via its N-methyl group, to Asp72. The hydroxyl group of the inhibitor makes a strong hydrogen bond (2.7 Å) with Glu199 [4]. ACh (gray) is shown for comparison.
Tacrine
(Cognex).
In the X-ray crystal structure of TcAChE/ complex which was determined at 2.8 Å resolution, the tacrine is seen (magenta) bound in the active site of TcAChE (1acj) [5]. ACh (gray) is shown for comparison.
See Tacrine.
Rivastigmine
(Exelon) is a carbamate inhibitor of AChE, and it is currenly used in therapy of Alzheimer's Disease. Rivastigmine (colored yellow) interacts with TcAChE (colored green) at the (1gqr). The carbamyl moiety of rivastigmine is to the active-site S200 Oγ. The second part of rivastigmine (the leaving group), NAP ((−)-S-3-[1-(dimethylamino)ethyl]phenol) is also held in the active-site gorge, but it is from the carbamyl moiety, hence, carbamylation took place. The of TcAChE/NAP (colored magenta) is known (1gqs). The TcAChE active-site residues which are interacting with NAP are colored violet. NAP is located in a similar region of TcAChE active site, but with different orientation than that of the NAP part (colored yellow) in the TcAChE/rivastigmine complex. Only H440 and F330 significantly change their side-chain conformations. of the TcAChE active sites in 4 different structures TcAChE/rivastigmine (1gqr), TcAChE/NAP (1gqs), native TcAChE (2ace), and TcAChE/VX (1vxr, TcAChE colored white and VX black) reveals that the conformation of H440 in the TcAChE/NAP structure is very similar its conformation in the native TcAChE (2ace), but the distance between H440 Nδ and E327 Oε is significantly longer in the TcAChE/rivastigmine and the TcAChE/VX complexes. This structural change disrupts the catalytic triad consisting of S200, E327, H440. This could explain the very slow kinetics of AChE reactivation after its inhibition by Rivastigmine [6].
The second generation of AD drugs - bivalent AChE inhibitors
The active site of consists of . First of them is the "catalytic anionic site" (CAS), which involves mentioned above catalytic triad (colored orange) and the conserved residues and also participating in ligands recognition. Another conserved residue (colored cyan) is situated at the second binding subsite, termed the "peripheral anionic site" (PAS), ~14 Å from CAS. Therefore, the ligands that will be able to interact with both these subsites, will be more potent AChE inhibitors in comparison to compounds interacting only with CAS (mentioned in the previous section "The first generation of AD drugs - monovalent AChE inhibitors"). One of the ways to produce such ligands is to introduce two active substances into one compound. If it is spatially necessary these subunits could be divided by alkyl linker with suitable length. For example, according to the strategy of the use of a bivalent ligand, the (RS)-(±)-tacrine-(10)-hupyridone ((R)-3 or (S)-3) was designed and synthesized. It consists of mentioned above (colored magenta), 10-carbon (yellow), and (red). The tacrine moiety of this inhibitor binds at the CAS, the linker spans the gorge, and the hupyridone moiety binds at the PAS (1zgb) [7]. There are also only PAS-binding AChE inhibitors, (magenta) is a good example of them. of the crystal structure of the edrophonium/TcAChE (CAS-binding inhibitor) (2ack) on the thioflavin T/TcAChE complex structure (2j3q) shows that these ligands' positions do not overlap. Of note is that Phe330, which is part of the CAS, is the single residue interacting with thioflavin T. This residue is the only one which significantly to avoid clashes in comparison to other CAS residues of the edrophonium/TcAChE complex [8] [9].
Compound 3
Described above, (abbreviated as ; colored red) is a CAS-binding inhibitor and it is currently used in therapy of Alzheimer's Disease under the trade name Razadyne. Conjugate of GAL through the (8 carbons, yellow) with a (blueviolet) called compound 3 has a larger affinity for AChE than that of GAL alone. This is similar to previously described cases of bivalent ligands (e.g. (RS)-(±)-tacrine-(10)-hupyridone). A comparison between /TcAChE (1w4l) and structure (1dx6) shows an identical binding mode of the GAL-moiety (transparent red) of compound 3 to that of GAL alone (blue) at the CAS. A PEG molecule (gray) is located at the active site of the galanthamine/TcAChE structure. The alkyl linker spans the active-site gorge and the phthalimido moiety of compound 3 is situated near Trp279 at the PAS. Compound 3 has higher affinity to TcAChE than GAL. This can be explained by the higher number of interactions between compound 3 (which interacts not only with residues within CAS but also within PAS) and TcAChE relative to GAL [10].
Aricept (donepezil, E2020)
(Donepezil) is one of the most interesting drugs that have been designed as AChE bivalent inhibitors. It was developed, synthesized and evaluated by the Eisai Company in Japan. These inhibitors were designed on the basis of QSAR studies prior to elucidation of the 3D structure of Torpedo californica AChE (TcAChE) (1ea5). It significantly enhances performance in animal models of cholinergic hypofunction and has a high affinity for AChE, binding to both electric eel and mouse AChE in the nanomolar range. The X-ray structure of the E2020-TcAChE complex (1eve) shows that E2020 has a along the active-site gorge, extending from the anionic subsite () of the active site, at the bottom, to the peripheral anionic site (), at the top, via aromatic stacking interactions with conserved aromatic acid residues. E2020 does not, however, interact directly with either the catalytic triad or the 'oxyanion hole' but only . The X-ray structure shows, a posteriori, that the design of E2020 took advantage of several important features of the active-site gorge of AChE, to produce a drug with both high affinity for AChE and a high degree of selectivity for AChE versus butyrylcholinesterase (BChE). It also delineates voids within the gorge that are not occupied by E2020 and could provide sites for potential modification of E2020 to produce drugs with improved pharmacological profiles [11].
See Treatments:AChE Inhibitor References.
See details of AChE-Aricept complex in various languages
1eve (German)
1eve (Hebrew)
1eve (Arabic)
1eve (Spanish)
1eve (Italian)
1eve (Chinese)
1eve (Turkish)
1eve (Russian)
Organophosphorus acid anhydride nerve agents
Organophosphorus (OP) acid anhydride nerve agents are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated.
As was mentioned above, AChE hydrolysizes the neurotransmitter , producing group. directly binds catalytic (via its nucleophilic Oγ atom). , O-(1,2,2-trimethylpropyl) methylphosphonofluoridate (fluorine atom is colored violet and phosphorus atom is colored darkmagenta), is one of the most toxic OPs. Soman inhibits AChE by to catalytic Ser200, . This process implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the ("aging") of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of , AChE can be by nucleophiles, such as pralidoxime (2-PAM), resulting in of the phosphonyl adduct from Ser200 Oγ.
At the (2wfz) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a red ball. The active site residues of the nonaged soman/TcAChE are colored yellow. The O2 atom of the (2wg0) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored pink. of the structures of the nonaged (2wfz) and aged (2wg0) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an after soman aging to TcAChE (2wg1) [12].
Structural and functional characterization of the interaction of the photosensitizing probe methylene blue with Torpedo californica acetylcholinesterase [13]
The photosensitizer, (colored in darkmagenta), generates singlet oxygen that irreversibly inhibits Torpedo californica acetylcholinesterase (TcAChE). In the dark, it inhibits reversibly.
The TcAChE active site consists of two binding subsites. One of them is the "catalytic anionic site" (CAS), which involves the catalytic triad (colored in orange) and the conserved residues which also participate in ligand recognition. Another conserved residue (colored in cyan) is situated at the second binding subsite, termed the "peripheral anionic site" (PAS), ~14 Å from CAS. (2j3q) is a good example of the PAS-binding AChE inhibitors. of the structure of known CAS-binding inhibitor edrophonium/TcAChE (2ack) on the thioflavin T/TcAChE complex structure (2j3q) shows that these [8] [9].
MB is a noncompetitive inhibitor of TcAChE, competing with reversible inhibitors directed at both ‘‘anionic’’ subsites, but a single site is involved in inhibition. The crystal structure reveals a , oriented down the gorge toward the CAS (2w9i); it is plausible that irreversible inhibition is associated with photooxidation of this residue and others within the active-site gorge. Superposition of the PAS regions of the MB/TcAChE (2w9i) and thioflavin T/TcAChE (2j3q) complexes reveals . As the conformation of TcAChE in the crystal structures of the two complexes is practically identical, only that of the MB/TcAChE structure (2w9i) is shown. The kinetic and spectroscopic data showing that inhibitors binding at the CAS can impede binding of MB are reconciled by docking studies showing that the , midway down the gorge, in the MB/TcAChE crystal structure, precludes simultaneous binding of a second MB at the CAS (2nd MB is colored blueviolet, Phe330 of the crystal structure is in orange and Phe330 of the modeled structure is in indigo). Conversely, binding of ligands at the CAS dislodges MB from its preferred locus at the PAS. The data presented demonstrate that TcAChE is a valuable model for understanding the molecular basis of local photooxidative damage.
For more details see
See also a model of African Malaria Mosquito Acetylcholinesterase.