We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Methotrexate
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
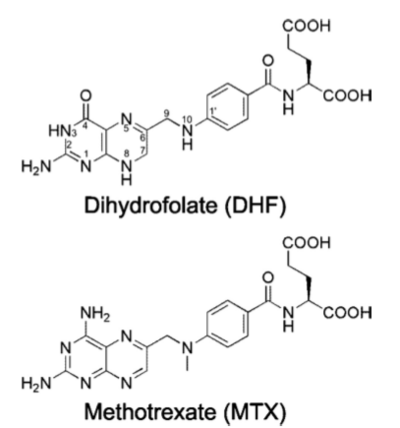
Human DHFR can be visualized as its <scene name='Sandbox_58/Biological_unit/1'>biological unit</scene>. DHFR contains 4 alpha helical regions and 8 beta sheets as can be seen in its <scene name='Sandbox_58/Secondary_structure_2w3m/1'>secondary structure</scene>. | Human DHFR can be visualized as its <scene name='Sandbox_58/Biological_unit/1'>biological unit</scene>. DHFR contains 4 alpha helical regions and 8 beta sheets as can be seen in its <scene name='Sandbox_58/Secondary_structure_2w3m/1'>secondary structure</scene>. | ||
| - | <scene name='42/420816/Secondary_structure_2w3m/ | + | <scene name='42/420816/Secondary_structure_2w3m/8'>Text To Be Displayed</scene> |
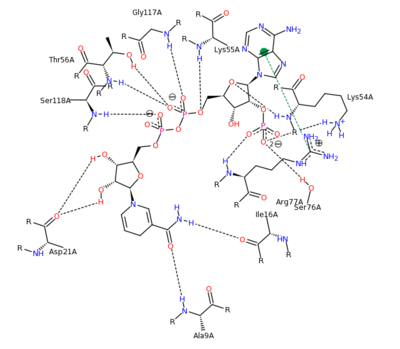
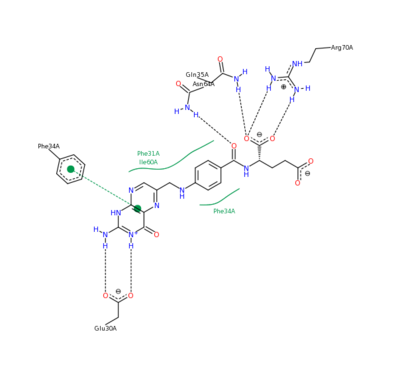
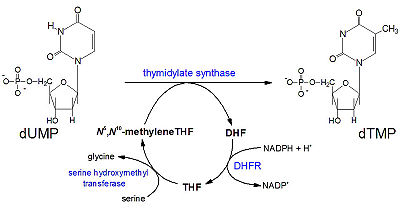
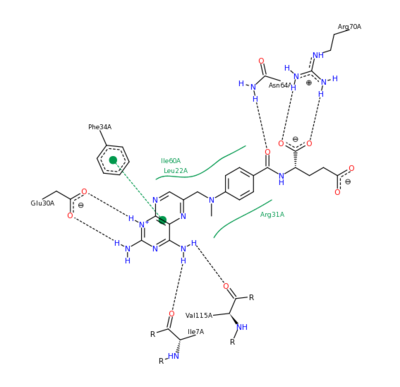
The <scene name='Sandbox_58/Acidic_basic/1'>acidic and basic residues</scene> can also be seen. Human DHFR catalyzes the reduction of dihydrofolic acid to tetrahydrofolic acid, with NADPH serving as the electron donor in this reaction. The <scene name='Sandbox_58/Active_site_2w3m-/1'>active site</scene> can be seen with the residues that facilitate substrate binding and reaction process. The red residues represent the active site amino acid side chains interacting with the substrate, and the blue amino acid side chains help bind NADPH, with both folate and NADPH represented in white. <scene name='Sandbox_58/Active_site_2w3m-/2'>NADPH and folate</scene> can both be seen interacting with the DHFR enzyme (folate surrounded by red sidechains, and NADPH surrounded by blue sidechains)<ref>Schnell JR, Dyson HJ, Wright PE (June 2004). "Structure, dynamics, and catalytic function of dihydrofolate reductase.". Annual Review of Biophysics and Biomolecular Structure 33: 119–40</ref>. | The <scene name='Sandbox_58/Acidic_basic/1'>acidic and basic residues</scene> can also be seen. Human DHFR catalyzes the reduction of dihydrofolic acid to tetrahydrofolic acid, with NADPH serving as the electron donor in this reaction. The <scene name='Sandbox_58/Active_site_2w3m-/1'>active site</scene> can be seen with the residues that facilitate substrate binding and reaction process. The red residues represent the active site amino acid side chains interacting with the substrate, and the blue amino acid side chains help bind NADPH, with both folate and NADPH represented in white. <scene name='Sandbox_58/Active_site_2w3m-/2'>NADPH and folate</scene> can both be seen interacting with the DHFR enzyme (folate surrounded by red sidechains, and NADPH surrounded by blue sidechains)<ref>Schnell JR, Dyson HJ, Wright PE (June 2004). "Structure, dynamics, and catalytic function of dihydrofolate reductase.". Annual Review of Biophysics and Biomolecular Structure 33: 119–40</ref>. | ||
Revision as of 11:38, 15 January 2017
| |||||||||||
References
- ↑ Methotrexate. (n.d.). UW Department of Orthopaedics and Sports Medicine - Patient Care. Retrieved March 10, 2011, from http://www.orthop.washington.edu/PatientCare/OurServices/Arthritis/Articles/Methotrexate.aspx
- ↑ Medical Pharmacology Topics. (n.d.). Angelfire: Welcome to Angelfire. Retrieved March 10, 2011, from http://www.angelfire.com/sc3/toxchick/medpharm/medpharm65.html
- ↑ Methotrexate. (n.d.). UW Department of Orthopaedics and Sports Medicine - Patient Care. Retrieved March 10, 2011, from http://www.orthop.washington.edu/PatientCare/OurServices/Arthritis/Articles/Methotrexate.aspx
- ↑ Schnell JR, Dyson HJ, Wright PE (June 2004). "Structure, dynamics, and catalytic function of dihydrofolate reductase.". Annual Review of Biophysics and Biomolecular Structure 33: 119–40
- ↑ DIHYDROFOLATE REDUCTASE COMPLEXED WITH METHOTREXATE. (n.d.). RCSB Protein Database. Retrieved March 10, 2011, from www.rcsb.org/pdb/results
- ↑ DIHYDROFOLATE REDUCTASE COMPLEXED WITH METHOTREXATE. (n.d.). RCSB Protein Database. Retrieved March 10, 2011, from www.rcsb.org/pdb/results
- ↑ DNA Synthesis - Replication: Chromatin Structure. (n.d.). The Medical Biochemistry Page. Retrieved March 10, 2011, from http://themedicalbiochemistrypage.org/dna.html
- ↑ Voet, D., Voet, J. G., & Pratt, C. W. (2008). Fundamentals of biochemistry: life at the molecular level (3rd ed.). Hoboken, NJ: Wiley.
- ↑ Rajagopalan, P. T. Ravi; Zhang, Zhiquan; McCourt, Lynn (2002). "Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics". Proceedings of the National Academy of Sciences 99 (21): 13481–6.
- ↑ Methotrexate and Folic Acid. (2006, September 3). Wikimedia Commons. Retrieved March 10, 2011, from commons.wikimedia.org/.png
- ↑ Matthews DA, Alden RA, Bolin JT, Freer ST, Hamlin R, Xuong N, Kraut J, Poe M, Williams M, Hoogsteen K (July 1977). "Dihydrofolate reductase: x-ray structure of the binary complex with methotrexate". Science 197 (4302): 452–455.
- ↑ DIHYDROFOLATE REDUCTASE COMPLEXED WITH METHOTREXATE. (n.d.). RCSB Protein Database. Retrieved March 10, 2011, from www.rcsb.org/pdb/results
- ↑ Enzymes. (n.d.). Oregon State University. Retrieved March 10, 2011, from http://oregonstate.edu/instruction/bb450/fall2010/lecture/enzymesoutline.html
- ↑ Volpato, J., Yachnin, B., & Blanchet, J. (2009). Multiple conformers in active site of human dihydrofolate reductase F31R/Q35E double mutant suggest structural basis for methotrexate resistance.. Journal Biol. Chem., 284, 20079-20089.
- ↑ DIHYDROFOLATE REDUCTASE COMPLEXED WITH METHOTREXATE. (n.d.). RCSB Protein Database. Retrieved March 10, 2011, from www.rcsb.org/pdb/results
- ↑ Methotrexate Information from Drugs.com. (n.d.). Drugs.com | Prescription Drugs - Information, Interactions & Side Effects. Retrieved March 10, 2011, from http://www.drugs.com/methotrexate.html
- ↑ Marks, J. W. (2008, January 8). Methotrexate. Medicine Net. Retrieved March 10, 2011, from www.medicinenet.com/methotrexate/article.htm
- ↑ Trexall. (2007, November 20). The RX List. Retrieved March 10, 2011, from www.rxlist.com/trexall-drug.htm
- ↑ Schwartza, S., & Borner, K. (2007). Glucarpidase (Carboxypeptidase G2) Intervention in Adult and Elderly Cancer Patients with Renal Dysfunction and Delayed Methotrexate Elimination After High-Dose Methotrexate Therapy. The Oncologist, 12(11), 1299-1308.
- ↑ Sirotnak, F., Dorick, D., & Moccio, D. (1978). Murine Tumor ModelsRescue Therapy in the L1210 Leukemia and Sarcoma 180 Optimization of High-Dose Methotrexate with Leucovorin . CANCER RESEARCH, 38, 345-353. Retrieved March 10, 2011, from cancerres.aacrjournals.org/content/38/2/345.full.pdf
- ↑ Methotrexate. (2010, September 1). CCO Formulary. Retrieved March 10, 2011, from www.cancercare.on.ca/pdfdrugs/methotre.pdf
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Joel L. Sussman, Daniel Kreider, David Canner, OCA