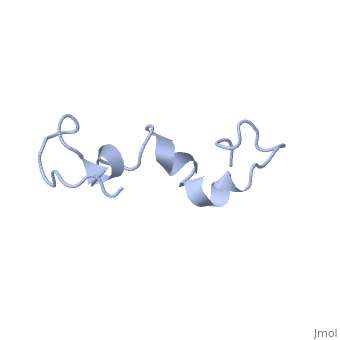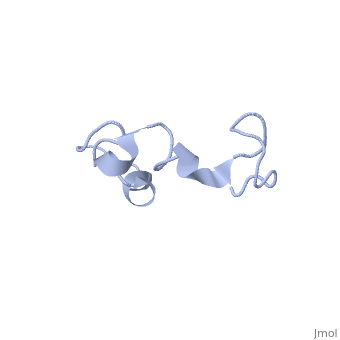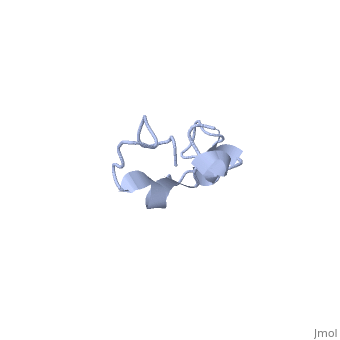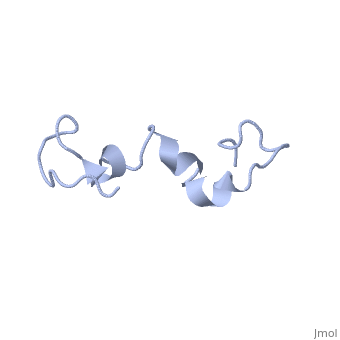This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Tau protein
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
==Structure and Function == | ==Structure and Function == | ||
| - | The human '''tau protein | + | The human '''tau protein''', encoded by chromosome 17q21, has a natively unfolded protein structure, which contributes to its flexibility and ability to stabilize functional microtubules <ref name="mandelkow">PMID: 22762014</ref>. Specifically, its primary structure, consisting of <scene name='71/716561/Primary_tau/1'>serines, threonines, aspartates, glutamates, lysines, arginines, prolines, and aromatics</scene>, is highly hydrophilic compared to other cytosolic proteins <ref name="mandelkow"/>. It has a predominantly acidic N-terminal region, a proline-rich middle region, and a relatively neutral C-terminal region <ref name="mandelkow"/>. |
Additionally, tau has a transient secondary structure of α-helices, β-pleated sheets, and a poly-proline II helix <ref name="mandelkow"/>. Tau does not resemble a globular protein, but has characteristics of a denatured, unfolded protein which contributes to its overall hydrophilicity <ref name="schweers">PMID: 7929085</ref>. It can also interact with other tau proteins to form <scene name='71/716561/Tau_aggregation/1'>aggregations</scene>. | Additionally, tau has a transient secondary structure of α-helices, β-pleated sheets, and a poly-proline II helix <ref name="mandelkow"/>. Tau does not resemble a globular protein, but has characteristics of a denatured, unfolded protein which contributes to its overall hydrophilicity <ref name="schweers">PMID: 7929085</ref>. It can also interact with other tau proteins to form <scene name='71/716561/Tau_aggregation/1'>aggregations</scene>. | ||
| Line 20: | Line 20: | ||
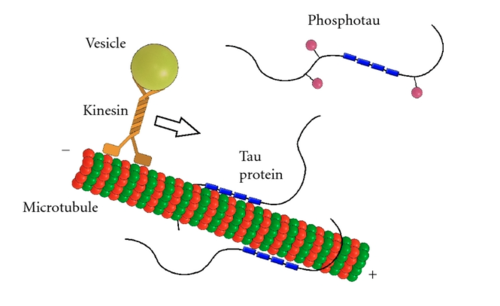
[[Image:PhosphorylatedTau.png|500px|thumb|Role of human tau in stabilization of microtubules through tubulin binding domains (blue). Phosphorylation (pink) of tau can directly regulate its interaction with microtubules and its role in axonal transport <ref name="Kolarova"/>]] | [[Image:PhosphorylatedTau.png|500px|thumb|Role of human tau in stabilization of microtubules through tubulin binding domains (blue). Phosphorylation (pink) of tau can directly regulate its interaction with microtubules and its role in axonal transport <ref name="Kolarova"/>]] | ||
| - | |||
==Disease Relation== | ==Disease Relation== | ||
| Line 30: | Line 29: | ||
Protein aggregation also exists in patients suffering from Parkinson’s disease, specifically within the substantia nigra of the midbrain <ref name="Ross">PMID: 15272267</ref>. Immunohistochemical studies reveal that abnormally phosphorylated tau proteins are partially involved in the development of Lewy bodies, protein aggregates, within the substantia nigra <ref name="Ishizawa">PMID: 12722831</ref>. Lewy bodies may be involved in the neurodegeneration of dopaminergic neurons within this area <ref name="Ross"/>. | Protein aggregation also exists in patients suffering from Parkinson’s disease, specifically within the substantia nigra of the midbrain <ref name="Ross">PMID: 15272267</ref>. Immunohistochemical studies reveal that abnormally phosphorylated tau proteins are partially involved in the development of Lewy bodies, protein aggregates, within the substantia nigra <ref name="Ishizawa">PMID: 12722831</ref>. Lewy bodies may be involved in the neurodegeneration of dopaminergic neurons within this area <ref name="Ross"/>. | ||
==3D Structures of tau protein== | ==3D Structures of tau protein== | ||
| + | [[Tau protein 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
| - | ==3D Structures of tau protein== | ||
| - | [[Tau protein 3D structures]] | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | |||
| - | [[6nwp]], [[6nwq]], [[6tjx]], [[6tjo]], [[6hre]], [[6hrf]] – hTau 1-441 – human - Cryo EM<br /> | ||
| - | [[1i8h]] – hTau peptide 541-553 + PIN1 WW domain - NMR<br /> | ||
| - | [[6n4p]] – hTau peptide 5-10<br /> | ||
| - | [[2on9]], [[4e0n]], [[4e0o]], [[4e0m]], [[5k7n]], [[3q9g]] – hTau peptide 305-311<br /> | ||
| - | [[5v5c]], [[5v5b]], [[6nk4]] – hTau peptide 592-597<br /> | ||
| - | [[4np8]], [[6odg]] – hTau peptide 623-628<br /> | ||
| - | [[5n5a]], [[5n5b]], [[5nvb]] – hTau peptide 571-607 - NMR<br /> | ||
| - | [[2mz7]] – hTau peptide 584-629 - NMR<br /> | ||
| - | [[6vha]], [[6vh7]], [[6vhl]] – hTau peptide 216-322 - Cryo EM<br /> | ||
| - | [[6qjh]], [[6qjm]], [[6qjp]], [[6qjq]] – hTau peptide 243-301 - Cryo EM<br /> | ||
| - | [[6gx5]] – hTau peptide 196-289 - Cryo EM<br /> | ||
| - | [[6vi3]] – hTau peptide 275-351 - Cryo EM<br /> | ||
| - | [[5o3o]], [[5o3t]], [[5o3l]] – hTau peptide 623-695 - Cryo EM<br /> | ||
| - | [[3ovl]] – hTau peptide 306-311 + Orange G<br /> | ||
| - | [[4glr]], [[4tqe]], [[5dmg]], [[5e2v]], [[5e2w]], [[5zv3]], [[6dc8]], [[6dc9]], [[6dca]], [[6dcw]], [[6h06]], [[6h0e]], [[6gk7]], [[6gk8]], [[2v17]], [[5mo3]], [[5mp1]], [[5mp3]], [[5mp5]], [[6bb4]], [[6lra]], [[6pxr]], [[6xli]] – hTau peptide + antibody<br /> | ||
| - | [[4fl5]], [[4y32]], [[4y3b]], [[4y5i]], [[5btv]], [[5hf3]], [[6fau]], [[6fav]], [[6faw]], [[6fbw]], [[6fby]], [[6fi4]], [[6fi5]], [[2btp]] – hTau peptide + 14-3-3 protein σ<br /> | ||
| - | [[6cvn]], [[6cvj]] – hTau peptide 198-399 + tubulin - Cryo EM<br /> | ||
| - | |||
==References== | ==References== | ||
Revision as of 09:41, 17 April 2022
References
- ↑ 1.0 1.1 1.2 1.3 Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006247. doi:, 10.1101/cshperspect.a006247. PMID:22762014 doi:http://dx.doi.org/10.1101/cshperspect.a006247
- ↑ 2.0 2.1 Schweers O, Schonbrunn-Hanebeck E, Marx A, Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994 Sep 30;269(39):24290-7. PMID:7929085
- ↑ 3.0 3.1 3.2 Lei P, Ayton S, Finkelstein DI, Adlard PA, Masters CL, Bush AI. Tau protein: relevance to Parkinson's disease. Int J Biochem Cell Biol. 2010 Nov;42(11):1775-8. doi:, 10.1016/j.biocel.2010.07.016. Epub 2010 Aug 1. PMID:20678581 doi:http://dx.doi.org/10.1016/j.biocel.2010.07.016
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Buee, L, Bussiere, T, Buee-Scherrer, V, Delacourte, A, Hof, PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 33:95-130 (2000). DOI: 10.1016/S0165-0173(00)00019-9
- ↑ 5.0 5.1 5.2 5.3 5.4 Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012:731526. doi: 10.1155/2012/731526. Epub 2012 May, 29. PMID:22690349 doi:http://dx.doi.org/10.1155/2012/731526
- ↑ Qurashi I, Collins J, Chaudhry I, Husain N. Promising use of minocycline augmentation with clozapine in treatment-resistant schizophrenia. J Psychopharmacol. 2014 Mar 19;28(7):707-708. PMID:24646811 doi:http://dx.doi.org/10.1177/0269881114527358
- ↑ 7.0 7.1 7.2 Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991 Nov;115(3):717-30. PMID:1918161
- ↑ Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004 Aug 13;279(33):34873-81. Epub 2004 Jun 9. PMID:15190058 doi:http://dx.doi.org/10.1074/jbc.M405131200
- ↑ Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004 Jul;10 Suppl:S10-7. PMID:15272267 doi:http://dx.doi.org/10.1038/nm1066
- ↑ Biernat J, Mandelkow EM, Schroter C, Lichtenberg-Kraag B, Steiner B, Berling B, Meyer H, Mercken M, Vandermeeren A, Goedert M, et al.. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992 Apr;11(4):1593-7. PMID:1563356
- ↑ Cohen TJ, Friedmann D, Hwang AW, Marmorstein R, Lee VM. The microtubule-associated tau protein has intrinsic acetyltransferase activity. Nat Struct Mol Biol. 2013 Jun;20(6):756-62. doi: 10.1038/nsmb.2555. Epub 2013 Apr, 28. PMID:23624859 doi:http://dx.doi.org/10.1038/nsmb.2555
- ↑ Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):7501-6. doi:, 10.1073/pnas.1504081112. Epub 2015 Jun 1. PMID:26034266 doi:http://dx.doi.org/10.1073/pnas.1504081112
- ↑ 13.0 13.1 Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004 Jul;10 Suppl:S10-7. PMID:15272267 doi:http://dx.doi.org/10.1038/nm1066
- ↑ Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003 Apr;62(4):389-97. PMID:12722831
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Madelyn Kasprzak, Jaime Prilusky